The First Law of Thermodynamics: Closed Systems Heat Transfer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3-1 Adiabatic Compression
Solution Physics 213 Problem 1 Week 3 Adiabatic Compression a) Last week, we considered the problem of isothermal compression: 1.5 moles of an ideal diatomic gas at temperature 35oC were compressed isothermally from a volume of 0.015 m3 to a volume of 0.0015 m3. The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final volume. Is the final temperature higher, lower, or the same as the in isothermal case? Sketch the adiabatic processes on the p-V diagram below and compute the final temperature. (Ignore vibrations of the molecules.) α α For an adiabatic process ViTi = VfTf , where α = 5/2 for the diatomic gas. In this case Ti = 273 o 1/α 2/5 K + 35 C = 308 K. We find Tf = Ti (Vi/Vf) = (308 K) (10) = 774 K. b) According to your diagram, is the final pressure greater, lesser, or the same as in the isothermal case? Explain why (i.e., what is the energy flow in each case?). Calculate the final pressure. We argued above that the final temperature is greater for the adiabatic process. Recall that p = nRT/ V for an ideal gas. We are given that the final volume is the same for the two processes. Since the final temperature is greater for the adiabatic process, the final pressure is also greater. We can do the problem numerically if we assume an idea gas with constant α. γ In an adiabatic processes, pV = constant, where γ = (α + 1) / α. -

Lecture 4: 09.16.05 Temperature, Heat, and Entropy
3.012 Fundamentals of Materials Science Fall 2005 Lecture 4: 09.16.05 Temperature, heat, and entropy Today: LAST TIME .........................................................................................................................................................................................2� State functions ..............................................................................................................................................................................2� Path dependent variables: heat and work..................................................................................................................................2� DEFINING TEMPERATURE ...................................................................................................................................................................4� The zeroth law of thermodynamics .............................................................................................................................................4� The absolute temperature scale ..................................................................................................................................................5� CONSEQUENCES OF THE RELATION BETWEEN TEMPERATURE, HEAT, AND ENTROPY: HEAT CAPACITY .......................................6� The difference between heat and temperature ...........................................................................................................................6� Defining heat capacity.................................................................................................................................................................6� -

The First Law of Thermodynamics for Closed Systems A) the Energy
Chapter 3: The First Law of Thermodynamics for Closed Systems a) The Energy Equation for Closed Systems We consider the First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle. Thus energy is transferred between the system and the surroundings in the form of heat and work, resulting in a change of internal energy of the system. Internal energy change can be considered as a measure of molecular activity associated with change of phase or temperature of the system and the energy equation is represented as follows: Heat (Q) Energy transferred across the boundary of a system in the form of heat always results from a difference in temperature between the system and its immediate surroundings. We will not consider the mode of heat transfer, whether by conduction, convection or radiation, thus the quantity of heat transferred during any process will either be specified or evaluated as the unknown of the energy equation. By convention, positive heat is that transferred from the surroundings to the system, resulting in an increase in internal energy of the system Work (W) In this course we consider three modes of work transfer across the boundary of a system, as shown in the following diagram: Source URL: http://www.ohio.edu/mechanical/thermo/Intro/Chapt.1_6/Chapter3a.html Saylor URL: http://www.saylor.org/me103#4.1 Attributed to: Israel Urieli www.saylor.org Page 1 of 7 In this course we are primarily concerned with Boundary Work due to compression or expansion of a system in a piston-cylinder device as shown above. -
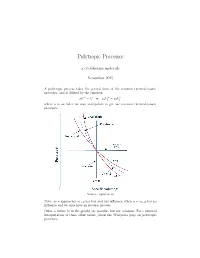
Polytropic Processes
Polytropic Processes a.cyclohexane.molecule November 2015 A polytropic process takes the general form of the common thermodynamic processes, and is defined by the equation n n n pV = C or p1V1 = p2V2 where n is an index we may manipulate to get our common thermodynamic processes. Source: nptel.ac.in Note: as n approaches 1, p has less and less influence; when n = 1, p has no influence and we thus have an isobaric process. Other n values (x in the graph) are possible, but not common. For a physical interpretation of these other values, check the Wikipedia page on polytropic processes. Because polytropic processes are general representations of thermodynamic pro- cesses, any quantities we calculate for a polytropic process will be a general equation for that quantity in all other thermodynamic processes. We proceed to find the work and heat associated with a polytropic process. We start with the definition of work: Z V2 w = − p dV V1 The polytropic equation pV n = C allows us to manipulate our formula for work in the following manner, as long as n 6= 1 V V Z 2 C Z 2 C V C V w = − dV = −C V −n dV = − V 1−n 2 = V 1−n 2 n V1 V1 V1 V V1 1 − n n − 1 Evaluating this expression, C CV 1−n − CV 1−n w = V 1−n − V 1−n = 2 1 n − 1 2 1 n − 1 n n We let the first C be p2V2 and the second C be p1V1 , such that the exponents cancel to obtain p V nV 1−n − p V nV 1−n p V − p V R(T − T ) w = 2 2 2 1 1 1 = 2 2 1 1 = 2 1 n − 1 n − 1 n − 1 where the last equality follows from the ideal gas law pV = RT and our deriva- tion for the work of a polytropic process is complete. -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

MECH 230 – Thermodynamics 1 Final Workbook Solutions
MECH 230 – Thermodynamics 1 Final Workbook Solutions CREATED BY JUSTIN BONAL MECH 230 Final Exam Workbook Contents 1.0 General Knowledge ........................................................................................................................... 1 1.1 Unit Analysis .................................................................................................................................. 1 1.2 Pressure ......................................................................................................................................... 1 2.0 Energies ............................................................................................................................................ 2 2.1 Work .............................................................................................................................................. 2 2.2 Internal Energy .............................................................................................................................. 3 2.3 Heat ............................................................................................................................................... 3 2.4 First Law of Thermodynamics ....................................................................................................... 3 3.0 Ideal gas ............................................................................................................................................ 4 3.1 Universal Gas Constant ................................................................................................................. -

Heat Transfer in Flat-Plate Solar Air-Heating Collectors
Advanced Computational Methods in Heat Transfer VI, C.A. Brebbia & B. Sunden (Editors) © 2000 WIT Press, www.witpress.com, ISBN 1-85312-818-X Heat transfer in flat-plate solar air-heating collectors Y. Nassar & E. Sergievsky Abstract All energy systems involve processes of heat transfer. Solar energy is obviously a field where heat transfer plays crucial role. Solar collector represents a heat exchanger, in which receive solar radiation, transform it to heat and transfer this heat to the working fluid in the collector's channel The radiation and convection heat transfer processes inside the collectors depend on the temperatures of the collector components and on the hydrodynamic characteristics of the working fluid. Economically, solar energy systems are at best marginal in most cases. In order to realize the potential of solar energy, a combination of better design and performance and of environmental considerations would be necessary. This paper describes the thermal behavior of several types of flat-plate solar air-heating collectors. 1 Introduction Nowadays the problem of utilization of solar energy is very important. By economic estimations, for the regions of annual incident solar radiation not less than 4300 MJ/m^ pear a year (i.e. lower 60 latitude), it will be possible to cover - by using an effective flat-plate solar collector- up to 25% energy demand in hot water supply systems and up to 75% in space heating systems. Solar collector is the main element of any thermal solar system. Besides of large number of scientific publications, on the problem of solar energy utilization, for today there is no common satisfactory technique to evaluate the thermal behaviors of solar systems, especially the local characteristics of the solar collector. -

Work and Energy Summary Sheet Chapter 6
Work and Energy Summary Sheet Chapter 6 Work: work is done when a force is applied to a mass through a displacement or W=Fd. The force and the displacement must be parallel to one another in order for work to be done. F (N) W =(Fcosθ)d F If the force is not parallel to The area of a force vs. the displacement, then the displacement graph + W component of the force that represents the work θ d (m) is parallel must be found. done by the varying - W d force. Signs and Units for Work Work is a scalar but it can be positive or negative. Units of Work F d W = + (Ex: pitcher throwing ball) 1 N•m = 1 J (Joule) F d W = - (Ex. catcher catching ball) Note: N = kg m/s2 • Work – Energy Principle Hooke’s Law x The work done on an object is equal to its change F = kx in kinetic energy. F F is the applied force. 2 2 x W = ΔEk = ½ mvf – ½ mvi x is the change in length. k is the spring constant. F Energy Defined Units Energy is the ability to do work. Same as work: 1 N•m = 1 J (Joule) Kinetic Energy Potential Energy Potential energy is stored energy due to a system’s shape, position, or Kinetic energy is the energy of state. motion. If a mass has velocity, Gravitational PE Elastic (Spring) PE then it has KE 2 Mass with height Stretch/compress elastic material Ek = ½ mv 2 EG = mgh EE = ½ kx To measure the change in KE Change in E use: G Change in ES 2 2 2 2 ΔEk = ½ mvf – ½ mvi ΔEG = mghf – mghi ΔEE = ½ kxf – ½ kxi Conservation of Energy “The total energy is neither increased nor decreased in any process. -

Specific Energy Limit and Its Influence on the Nature of Black Holes Javier Viaña
Specific Energy Limit and its Influence on the Nature of Black Holes Javier Viaña To cite this version: Javier Viaña. Specific Energy Limit and its Influence on the Nature of Black Holes. 2021. hal- 03322333 HAL Id: hal-03322333 https://hal.archives-ouvertes.fr/hal-03322333 Preprint submitted on 19 Aug 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. 16th of August of 2021 Specific Energy Limit and its Influence on the Nature of Black Holes Javier Viaña [0000-0002-0563-784X] University of Cincinnati, Cincinnati OH 45219, USA [email protected] What if the universe has a limit on the amount of energy that a certain mass can have? This article explores this possibility and suggests a theory for the creation and nature of black holes based on an energetic limit. The Specific Energy Limit Energy is an extensive property, and we know that as we add more mass to a given system, we can easily increase its energy. Specific energy on the other hand is an intensive property. It is defined as the energy divided by the mass and it is measured in units of J/kg. -

Energy and the Hydrogen Economy
Energy and the Hydrogen Economy Ulf Bossel Fuel Cell Consultant Morgenacherstrasse 2F CH-5452 Oberrohrdorf / Switzerland +41-56-496-7292 and Baldur Eliasson ABB Switzerland Ltd. Corporate Research CH-5405 Baden-Dättwil / Switzerland Abstract Between production and use any commercial product is subject to the following processes: packaging, transportation, storage and transfer. The same is true for hydrogen in a “Hydrogen Economy”. Hydrogen has to be packaged by compression or liquefaction, it has to be transported by surface vehicles or pipelines, it has to be stored and transferred. Generated by electrolysis or chemistry, the fuel gas has to go through theses market procedures before it can be used by the customer, even if it is produced locally at filling stations. As there are no environmental or energetic advantages in producing hydrogen from natural gas or other hydrocarbons, we do not consider this option, although hydrogen can be chemically synthesized at relative low cost. In the past, hydrogen production and hydrogen use have been addressed by many, assuming that hydrogen gas is just another gaseous energy carrier and that it can be handled much like natural gas in today’s energy economy. With this study we present an analysis of the energy required to operate a pure hydrogen economy. High-grade electricity from renewable or nuclear sources is needed not only to generate hydrogen, but also for all other essential steps of a hydrogen economy. But because of the molecular structure of hydrogen, a hydrogen infrastructure is much more energy-intensive than a natural gas economy. In this study, the energy consumed by each stage is related to the energy content (higher heating value HHV) of the delivered hydrogen itself. -

Chapter 6. Time Evolution in Quantum Mechanics
6. Time Evolution in Quantum Mechanics 6.1 Time-dependent Schrodinger¨ equation 6.1.1 Solutions to the Schr¨odinger equation 6.1.2 Unitary Evolution 6.2 Evolution of wave-packets 6.3 Evolution of operators and expectation values 6.3.1 Heisenberg Equation 6.3.2 Ehrenfest’s theorem 6.4 Fermi’s Golden Rule Until now we used quantum mechanics to predict properties of atoms and nuclei. Since we were interested mostly in the equilibrium states of nuclei and in their energies, we only needed to look at a time-independent description of quantum-mechanical systems. To describe dynamical processes, such as radiation decays, scattering and nuclear reactions, we need to study how quantum mechanical systems evolve in time. 6.1 Time-dependent Schro¨dinger equation When we first introduced quantum mechanics, we saw that the fourth postulate of QM states that: The evolution of a closed system is unitary (reversible). The evolution is given by the time-dependent Schrodinger¨ equation ∂ ψ iI | ) = ψ ∂t H| ) where is the Hamiltonian of the system (the energy operator) and I is the reduced Planck constant (I = h/H2π with h the Planck constant, allowing conversion from energy to frequency units). We will focus mainly on the Schr¨odinger equation to describe the evolution of a quantum-mechanical system. The statement that the evolution of a closed quantum system is unitary is however more general. It means that the state of a system at a later time t is given by ψ(t) = U(t) ψ(0) , where U(t) is a unitary operator. -

Heat Transfer (Heat and Thermodynamics)
Heat Transfer (Heat and Thermodynamics) Wasif Zia, Sohaib Shamim and Muhammad Sabieh Anwar LUMS School of Science and Engineering August 7, 2011 If you put one end of a spoon on the stove and wait for a while, your nger tips start feeling the burn. So how do you explain this simple observation in terms of physics? Heat is generally considered to be thermal energy in transit, owing between two objects that are kept at di erent temperatures. Thermodynamics is mainly con- cerned with objects in a state of equilibrium, while the subject of heat transfer probes matter in a state of disequilibrium. Heat transfer is a beautiful and astound- ingly rich subject. For example, heat transfer is inextricably linked with atomic and molecular vibrations; marrying thermal physics with solid state physics|the study of the structure of matter. We all know that owing matter (such as air) in contact with a heated object can help `carry the heat away'. The motion of the uid, its turbulence, the ow pattern and the shape, size and surface of the object can have a pronounced e ect on how heat is transferred. These heat ow mechanisms are also an essential part of our ventilation and air conditioning mechanisms, adding comfort to our lives. Importantly, without heat exchange in power plants it is impossible to think of any power generation, without heat transfer the internal combustion engine could not drive our automobiles and without it, we would not be able to use our computer for long and do lengthy experiments (like this one!), without overheating and frying our electronics.