Feeling Joules and Watts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Locomotive Dedication Ceremony
The American Society of Mechanical Engineers REGIONAL MECHANICAL ENGINEERING HERITAGE COLLECTION LOCOMOTIVE DEDICATION CEREMONY Kenefick Park • Omaha, Nebraska June 7, 1994 Locomotives 4023 and 6900 are examples of the world’s largest motive power in the steam and diesel eras. These locomotives are on permanent display at Kenefick Park, which was established in 1989 in honor of noted former Union Pacific chairman, John C. Kenefick. The 4023 was one of twenty-five famous “Big Boy” type simple articulated locomotives Locomotive 4023 was a feature display lauded in the industry and press as the highest horsepower, heaviest and longest steam at the Omaha Shops locomotives ever built, developing seven thousand horsepower at their seventy miles until being moved to per hour design speed. Kenefick Park. 2 World’s largest The Big Boy type was designed at the Omaha headquarters of Union Pacific under the single unit diesel personal direction of the road’s noted mechanical head, Otto Jabelmann. The original locomotives required four axle trucks to distribute twenty locomotives of this type were built by American Locomotive Company in their heavy weight and Schenectady, New York, in the fall of 1941. They were built in preparation for the nation’s keep within track probable entry into World War II because no proven diesel freight locomotive was yet loading limits. in production. These 4-8-8-4 type locomotives were specifically designed to haul fast, heavy eastbound freight trains between Ogden, Utah, and Green River, Wyoming, over the 1.14 percent eastbound grade. The 4023 was one of five additional units built in 1944 under govern- ment authority in preparation for a twenty-five percent increase in traffic due to the shift from European to Pacific war operations. -

Abstract 1. Introduction 2. Robert Stirling
Stirling Stuff Dr John S. Reid, Department of Physics, Meston Building, University of Aberdeen, Aberdeen AB12 3UE, Scotland Abstract Robert Stirling’s patent for what was essentially a new type of engine to create work from heat was submitted in 1816. Its reception was underwhelming and although the idea was sporadically developed, it was eclipsed by the steam engine and, later, the internal combustion engine. Today, though, the environmentally favourable credentials of the Stirling engine principles are driving a resurgence of interest, with modern designs using modern materials. These themes are woven through a historically based narrative that introduces Robert Stirling and his background, a description of his patent and the principles behind his engine, and discusses the now popular model Stirling engines readily available. These topical models, or alternatives made ‘in house’, form a good platform for investigating some of the thermodynamics governing the performance of engines in general. ---------------------------------------------------------------------------------------------------------------- 1. Introduction 2016 marks the bicentenary of the submission of Robert Stirling’s patent that described heat exchangers and the technology of the Stirling engine. James Watt was still alive in 1816 and his steam engine was gaining a foothold in mines, in mills, in a few goods railways and even in pioneering ‘steamers’. Who needed another new engine from another Scot? The Stirling engine is a markedly different machine from either the earlier steam engine or the later internal combustion engine. For reasons to be explained, after a comparatively obscure two centuries the Stirling engine is attracting new interest, for it has environmentally friendly credentials for an engine. This tribute introduces the man, his patent, the engine and how it is realised in example models readily available on the internet. -
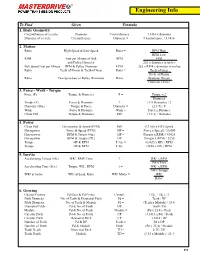
Engineering Info
Engineering Info To Find Given Formula 1. Basic Geometry Circumference of a circle Diameter Circumference = 3.1416 x diameter Diameter of a circle Circumference Diameter = Circumference / 3.1416 2. Motion Ratio High Speed & Low Speed Ratio = RPM High RPM Low RPM Feet per Minute of Belt RPM = FPM and Pulley Diameter .262 x diameter in inches Belt Speed Feet per Minute RPM & Pulley Diameter FPM = .262 x RPM x diameter in inches Ratio Teeth of Pinion & Teeth of Gear Ratio = Teeth of Gear Teeth of Pinion Ratio Two Sprockets or Pulley Diameters Ratio = Diameter Driven Diameter Driver 3. Force - Work - Torque Force (F) Torque & Diameter F = Torque x 2 Diameter Torque (T) Force & Diameter T = ( F x Diameter) / 2 Diameter (Dia.) Torque & Force Diameter = (2 x T) / F Work Force & Distance Work = Force x Distance Chain Pull Torque & Diameter Pull = (T x 2) / Diameter 4. Power Chain Pull Horsepower & Speed (FPM) Pull = (33,000 x HP)/ Speed Horsepower Force & Speed (FPM) HP = (Force x Speed) / 33,000 Horsepower RPM & Torque (#in.) HP = (Torque x RPM) / 63025 Horsepower RPM & Torque (#ft.) HP = (Torque x RPM) / 5250 Torque HP & RPM T #in. = (63025 x HP) / RPM Torque HP & RPM T #ft. = (5250 x HP) / RPM 5. Inertia Accelerating Torque (#ft.) WK2, RMP, Time T = WK2 x RPM 308 x Time Accelerating Time (Sec.) Torque, WK2, RPM t = WK2 x RPM 308 x Torque WK2 at motor WK2 at Load, Ratio WK2 Motor = WK2 Ratio2 6. Gearing Gearset Centers Pd Gear & Pd Pinion Centers = ( PdG + PdP ) / 2 Pitch Diameter No. of Teeth & Diametral Pitch Pd = Teeth / DP Pitch Diameter No. -

Champ Math Study Guide Indesign
Champions of Mathematics — Study Guide — Questions and Activities Page 1 Copyright © 2001 by Master Books, Inc. All rights reserved. This publication may be reproduced for educational purposes only. BY JOHN HUDSON TINER To get the most out of this book, the following is recommended: Each chapter has questions, discussion ideas, research topics, and suggestions for further reading to improve students’ reading, writing, and thinking skills. The study guide shows the relationship of events in Champions of Mathematics to other fields of learning. The book becomes a springboard for exploration in other fields. Students who enjoy literature, history, art, or other subjects will find interesting activities in their fields of interest. Parents will find that the questions and activities enhance their investments in the Champion books because children of different age levels can use them. The questions with answers are designed for younger readers. Questions are objective and depend solely on the text of the book itself. The questions are arranged in the same order as the content of each chapter. A student can enjoy the book and quickly check his or her understanding and comprehension by the challenge of answering the questions. The activities are designed to serve as supplemental material for older students. The activities require greater knowledge and research skills. An older student (or the same student three or four years later) can read the book and do the activities in depth. CHAPTER 1 QUESTIONS 1. A B C D — Pythagoras was born on an island in the (A. Aegean Sea B. Atlantic Ocean C. Caribbean Sea D. -

3.Joule's Experiments
The Force of Gravity Creates Energy: The “Work” of James Prescott Joule http://www.bookrags.com/biography/james-prescott-joule-wsd/ James Prescott Joule (1818-1889) was the son of a successful British brewer. He tinkered with the tools of his father’s trade (particularly thermometers), and despite never earning an undergraduate degree, he was able to answer two rather simple questions: 1. Why is the temperature of the water at the bottom of a waterfall higher than the temperature at the top? 2. Why does an electrical current flowing through a conductor raise the temperature of water? In order to adequately investigate these questions on our own, we need to first define “temperature” and “energy.” Second, we should determine how the measurement of temperature can relate to “heat” (as energy). Third, we need to find relationships that might exist between temperature and “mechanical” energy and also between temperature and “electrical” energy. Definitions: Before continuing, please write down what you know about temperature and energy below. If you require more space, use the back. Temperature: Energy: We have used the concept of gravity to show how acceleration of freely falling objects is related mathematically to distance, time, and speed. We have also used the relationship between net force applied through a distance to define “work” in the Harvard Step Test. Now, through the work of Joule, we can equate the concepts of “work” and “energy”: Energy is the capacity of a physical system to do work. Potential energy is “stored” energy, kinetic energy is “moving” energy. One type of potential energy is that induced by the gravitational force between two objects held at a distance (there are other types of potential energy, including electrical, magnetic, chemical, nuclear, etc). -

An Atomic Physics Perspective on the New Kilogram Defined by Planck's Constant
An atomic physics perspective on the new kilogram defined by Planck’s constant (Wolfgang Ketterle and Alan O. Jamison, MIT) (Manuscript submitted to Physics Today) On May 20, the kilogram will no longer be defined by the artefact in Paris, but through the definition1 of Planck’s constant h=6.626 070 15*10-34 kg m2/s. This is the result of advances in metrology: The best two measurements of h, the Watt balance and the silicon spheres, have now reached an accuracy similar to the mass drift of the ur-kilogram in Paris over 130 years. At this point, the General Conference on Weights and Measures decided to use the precisely measured numerical value of h as the definition of h, which then defines the unit of the kilogram. But how can we now explain in simple terms what exactly one kilogram is? How do fixed numerical values of h, the speed of light c and the Cs hyperfine frequency νCs define the kilogram? In this article we give a simple conceptual picture of the new kilogram and relate it to the practical realizations of the kilogram. A similar change occurred in 1983 for the definition of the meter when the speed of light was defined to be 299 792 458 m/s. Since the second was the time required for 9 192 631 770 oscillations of hyperfine radiation from a cesium atom, defining the speed of light defined the meter as the distance travelled by light in 1/9192631770 of a second, or equivalently, as 9192631770/299792458 times the wavelength of the cesium hyperfine radiation. -

History of a Forgotten Engine Alex Cannella, News Editor
POWER PLAY History of a Forgotten Engine Alex Cannella, News Editor In 2017, there’s more variety to be found un- der the hood of a car than ever. Electric, hybrid and internal combustion engines all sit next to a range of trans- mission types, creating an ever-increasingly complex evolu- tionary web of technology choices for what we put into our automobiles. But every evolutionary tree has a few dead end branches that ended up never going anywhere. One such branch has an interesting and somewhat storied history, but it’s a history that’s been largely forgotten outside of columns describing quirky engineering marvels like this one. The sleeve-valve engine was an invention that came at the turn of the 20th century and saw scattered use between its inception and World War II. But afterwards, it fell into obscurity, outpaced (By Andy Dingley (scanner) - Scan from The Autocar (Ninth edition, circa 1919) Autocar Handbook, London: Iliffe & Sons., pp. p. 38,fig. 21, Public Domain, by the poppet valves we use in engines today that, ironically, https://commons.wikimedia.org/w/index.php?curid=8771152) it was initially developed to replace. Back when the sleeve-valve engine was first developed, through the economic downturn, and by the time the econ- the poppet valves in internal combustion engines were ex- omy was looking up again, poppet valve engines had caught tremely noisy contraptions, a concern that likely sounds fa- up to the sleeve-valve and were quickly becoming just as miliar to anyone in the automotive industry today. Charles quiet and efficient. -

Work and Energy Summary Sheet Chapter 6
Work and Energy Summary Sheet Chapter 6 Work: work is done when a force is applied to a mass through a displacement or W=Fd. The force and the displacement must be parallel to one another in order for work to be done. F (N) W =(Fcosθ)d F If the force is not parallel to The area of a force vs. the displacement, then the displacement graph + W component of the force that represents the work θ d (m) is parallel must be found. done by the varying - W d force. Signs and Units for Work Work is a scalar but it can be positive or negative. Units of Work F d W = + (Ex: pitcher throwing ball) 1 N•m = 1 J (Joule) F d W = - (Ex. catcher catching ball) Note: N = kg m/s2 • Work – Energy Principle Hooke’s Law x The work done on an object is equal to its change F = kx in kinetic energy. F F is the applied force. 2 2 x W = ΔEk = ½ mvf – ½ mvi x is the change in length. k is the spring constant. F Energy Defined Units Energy is the ability to do work. Same as work: 1 N•m = 1 J (Joule) Kinetic Energy Potential Energy Potential energy is stored energy due to a system’s shape, position, or Kinetic energy is the energy of state. motion. If a mass has velocity, Gravitational PE Elastic (Spring) PE then it has KE 2 Mass with height Stretch/compress elastic material Ek = ½ mv 2 EG = mgh EE = ½ kx To measure the change in KE Change in E use: G Change in ES 2 2 2 2 ΔEk = ½ mvf – ½ mvi ΔEG = mghf – mghi ΔEE = ½ kxf – ½ kxi Conservation of Energy “The total energy is neither increased nor decreased in any process. -

Active Short Circuit - Chassis Short Characterization and Potential Mitigation Technique for the MMRTG
Jet Propulsion Laboratory California Institute of Technology Multi-Mission RTG Active Short Circuit - Chassis Short Characterization and Potential Mitigation Technique for the MMRTG February 25, 2015 Gary Bolotin1 Nicholas Keyawa1 1Jet Propulsion Laboratory, California Institute of Technology 1 Jet Propulsion Laboratory California Institute of Technology Agenda Multi-Mission RTG • Introduction – MMRTG – Internal MMRTG Chassis Shorts • Active Short Circuit Purpose • Active Short Circuit Theory • Active Short Design and Component Layout • Conclusion and Future Work 2 Jet Propulsion Laboratory California Institute of Technology Introduction – MMRTG Multi-Mission RTG • The Multi-Mission Radioisotope Thermoelectric Generator (MMRTG) utilizes a combination of PbTe, PbSnTe, and TAGS thermoelectric couples to produce electric current from the heat generated by the radioactive decay of plutonium – 238. 3 Jet Propulsion Laboratory Introduction – Internal MMRTG Chassis California Institute of Technology Shorts Multi-Mission RTG • Shorts inside the MMRTG between the electrical power circuit and the MMRTG chassis have been detected via isolation checks and/or changes in the bus balance voltage. • Example location of short shown below MMRTG Chassis Internal MMRTG Short 4 Jet Propulsion Laboratory California Institute of Technology Active Short Circuit Purpose Multi-Mission RTG • The leading hypothesis suggests that the FOD which is causing the internal shorts are extremely small pieces of material that could potentially melt and/or sublimate away given a sufficient amount of current. • By inducing a controlled second short (in the presence of an internal MMRTG chassis short), a significant amount of current flow can be generated to achieve three main design goals: 1) Measure and characterize the MMRTG internal short to chassis, 2) Safely determine if the MMRTG internal short can be cleared in the presence of another controlled short 3) Quantify the amount of energy required to clear the MMRTG internal short. -

Re-Examining the Role of Nuclear Fusion in a Renewables-Based Energy Mix
Re-examining the Role of Nuclear Fusion in a Renewables-Based Energy Mix T. E. G. Nicholasa,∗, T. P. Davisb, F. Federicia, J. E. Lelandc, B. S. Patela, C. Vincentd, S. H. Warda a York Plasma Institute, Department of Physics, University of York, Heslington, York YO10 5DD, UK b Department of Materials, University of Oxford, Parks Road, Oxford, OX1 3PH c Department of Electrical Engineering and Electronics, University of Liverpool, Liverpool, L69 3GJ, UK d Centre for Advanced Instrumentation, Department of Physics, Durham University, Durham DH1 3LS, UK Abstract Fusion energy is often regarded as a long-term solution to the world's energy needs. However, even after solving the critical research challenges, engineer- ing and materials science will still impose significant constraints on the char- acteristics of a fusion power plant. Meanwhile, the global energy grid must transition to low-carbon sources by 2050 to prevent the worst effects of climate change. We review three factors affecting fusion's future trajectory: (1) the sig- nificant drop in the price of renewable energy, (2) the intermittency of renewable sources and implications for future energy grids, and (3) the recent proposition of intermediate-level nuclear waste as a product of fusion. Within the scenario assumed by our premises, we find that while there remains a clear motivation to develop fusion power plants, this motivation is likely weakened by the time they become available. We also conclude that most current fusion reactor designs do not take these factors into account and, to increase market penetration, fu- sion research should consider relaxed nuclear waste design criteria, raw material availability constraints and load-following designs with pulsed operation. -

EM Waves, Ray Optics, Optical Instruments Mar
Gen. Phys. II Exam 3 - Chs. 24,25,26 - EM Waves, Ray Optics, Optical Instruments Mar. 26, 2018 Rec. Time Name For full credit, make your work clear. Show formulas used, essential steps, and results with correct units and significant figures. Points shown in parenthesis. For TF and MC, choose the best answer. OpenStax Ch. 24 - Electromagnetic Waves 1. (3) Which type of electromagnetic (EM) waves has the highest frequency in vacuum? a. x-rays. b. infrared. c. red light. d. blue light. e. ultraviolet. f. AM radio. g. all tie. 2. (3) An EM wave is traveling vertically upward with its magnetic field vector oscillating north-south. Its electric field vector is oscillating a. north-south. b. east-west. c. vertically up and down. 3. (3) The first physicist to confirm the generation and detection of EM waves by using LC oscillator circuits was a. Alexander Bell. b. James Watt. c. Andr´e-Marie Amp`ere. d. Heinrich Hertz. e. Carl Friedrich Gauss. 4. (3) TF In vacuum, electromagnetic waves of higher frequencies travel faster than lower frequencies. 5. (3) TF EM waves in vacuum can be considered to be transverse waves. 6. (3) TF Earth's ozone layer is important in blocking dangerous infrared light from the sun. 7. (3) Which physical effect did James Clerk Maxwell add into the equations of electromagnetism that carry his name, based on theoretical reasoning? a. changing magnetic fields produce electric fields. b. changing electric fields produce magnetic fields. c. moving electric charges produce magnetic fields. d. moving electric charges experience magnetic forces. -

Guide for the Use of the International System of Units (SI)
Guide for the Use of the International System of Units (SI) m kg s cd SI mol K A NIST Special Publication 811 2008 Edition Ambler Thompson and Barry N. Taylor NIST Special Publication 811 2008 Edition Guide for the Use of the International System of Units (SI) Ambler Thompson Technology Services and Barry N. Taylor Physics Laboratory National Institute of Standards and Technology Gaithersburg, MD 20899 (Supersedes NIST Special Publication 811, 1995 Edition, April 1995) March 2008 U.S. Department of Commerce Carlos M. Gutierrez, Secretary National Institute of Standards and Technology James M. Turner, Acting Director National Institute of Standards and Technology Special Publication 811, 2008 Edition (Supersedes NIST Special Publication 811, April 1995 Edition) Natl. Inst. Stand. Technol. Spec. Publ. 811, 2008 Ed., 85 pages (March 2008; 2nd printing November 2008) CODEN: NSPUE3 Note on 2nd printing: This 2nd printing dated November 2008 of NIST SP811 corrects a number of minor typographical errors present in the 1st printing dated March 2008. Guide for the Use of the International System of Units (SI) Preface The International System of Units, universally abbreviated SI (from the French Le Système International d’Unités), is the modern metric system of measurement. Long the dominant measurement system used in science, the SI is becoming the dominant measurement system used in international commerce. The Omnibus Trade and Competitiveness Act of August 1988 [Public Law (PL) 100-418] changed the name of the National Bureau of Standards (NBS) to the National Institute of Standards and Technology (NIST) and gave to NIST the added task of helping U.S.