Law of Conversation of Energy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
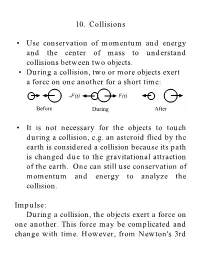
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Modeling Conservation of Mass
Modeling Conservation of Mass How is mass conserved (protected from loss)? Imagine an evening campfire. As the wood burns, you notice that the logs have become a small pile of ashes. What happened? Was the wood destroyed by the fire? A scientific principle called the law of conservation of mass states that matter is neither created nor destroyed. So, what happened to the wood? Think back. Did you observe smoke rising from the fire? When wood burns, atoms in the wood combine with oxygen atoms in the air in a chemical reaction called combustion. The products of this burning reaction are ashes as well as the carbon dioxide and water vapor in smoke. The gases escape into the air. We also know from the law of conservation of mass that the mass of the reactants must equal the mass of all the products. How does that work with the campfire? mass – a measure of how much matter is present in a substance law of conservation of mass – states that the mass of all reactants must equal the mass of all products and that matter is neither created nor destroyed If you could measure the mass of the wood and oxygen before you started the fire and then measure the mass of the smoke and ashes after it burned, what would you find? The total mass of matter after the fire would be the same as the total mass of matter before the fire. Therefore, matter was neither created nor destroyed in the campfire; it just changed form. The same atoms that made up the materials before the reaction were simply rearranged to form the materials left after the reaction. -

The First Law of Thermodynamics for Closed Systems A) the Energy
Chapter 3: The First Law of Thermodynamics for Closed Systems a) The Energy Equation for Closed Systems We consider the First Law of Thermodynamics applied to stationary closed systems as a conservation of energy principle. Thus energy is transferred between the system and the surroundings in the form of heat and work, resulting in a change of internal energy of the system. Internal energy change can be considered as a measure of molecular activity associated with change of phase or temperature of the system and the energy equation is represented as follows: Heat (Q) Energy transferred across the boundary of a system in the form of heat always results from a difference in temperature between the system and its immediate surroundings. We will not consider the mode of heat transfer, whether by conduction, convection or radiation, thus the quantity of heat transferred during any process will either be specified or evaluated as the unknown of the energy equation. By convention, positive heat is that transferred from the surroundings to the system, resulting in an increase in internal energy of the system Work (W) In this course we consider three modes of work transfer across the boundary of a system, as shown in the following diagram: Source URL: http://www.ohio.edu/mechanical/thermo/Intro/Chapt.1_6/Chapter3a.html Saylor URL: http://www.saylor.org/me103#4.1 Attributed to: Israel Urieli www.saylor.org Page 1 of 7 In this course we are primarily concerned with Boundary Work due to compression or expansion of a system in a piston-cylinder device as shown above. -

Chapter 6. Time Evolution in Quantum Mechanics
6. Time Evolution in Quantum Mechanics 6.1 Time-dependent Schrodinger¨ equation 6.1.1 Solutions to the Schr¨odinger equation 6.1.2 Unitary Evolution 6.2 Evolution of wave-packets 6.3 Evolution of operators and expectation values 6.3.1 Heisenberg Equation 6.3.2 Ehrenfest’s theorem 6.4 Fermi’s Golden Rule Until now we used quantum mechanics to predict properties of atoms and nuclei. Since we were interested mostly in the equilibrium states of nuclei and in their energies, we only needed to look at a time-independent description of quantum-mechanical systems. To describe dynamical processes, such as radiation decays, scattering and nuclear reactions, we need to study how quantum mechanical systems evolve in time. 6.1 Time-dependent Schro¨dinger equation When we first introduced quantum mechanics, we saw that the fourth postulate of QM states that: The evolution of a closed system is unitary (reversible). The evolution is given by the time-dependent Schrodinger¨ equation ∂ ψ iI | ) = ψ ∂t H| ) where is the Hamiltonian of the system (the energy operator) and I is the reduced Planck constant (I = h/H2π with h the Planck constant, allowing conversion from energy to frequency units). We will focus mainly on the Schr¨odinger equation to describe the evolution of a quantum-mechanical system. The statement that the evolution of a closed quantum system is unitary is however more general. It means that the state of a system at a later time t is given by ψ(t) = U(t) ψ(0) , where U(t) is a unitary operator. -

Key Concepts: Conservation of Mass, Momentum, Energy Fluid: a Material
Key concepts: Conservation of mass, momentum, energy Fluid: a material that deforms continuously and permanently under the application of a shearing stress, no matter how small. Fluids are either gases or liquids. (Under very specialized conditions, a phase of intermediate properties can be stable, but we won’t consider that possibility.) In liquids, the molecules are relatively closely spaced, allowing the magnitude of their (attractive, electrically-based) interaction energy to be of the same magnitude as their kinetic energy. As a result, they exist as a loose collection of clusters. In gases, the molecules are much more widely separated, so the kinetic energy (at a given temperature, identical to that in the liquid) is far greater than the interaction energy (much less than in the liquid), and molecule do not form clusters. In a liquid, the molecules themselves typically occupy a few percent of the total space available; in a gas, they occupy a few thousandths of a percent. Nevertheless, for our purposes, all fluids are considered to be continua (no voids or holes).The absence of significant intermolecular attraction allows gases to fill whatever volume is available to them, whereas the presence of such attraction in liquids prevents them from doing so. The attractive forces in liquid water are unusually strong, compared to other liquids. Properties of Fluids: m Density is mass/volume: ρ = . The density of liquid water is V 3 o −3 3 ~1.0 kg/m ; that of air at 20 C is ~1.2x10 kg/m . mg W Specific weight is weight/volume: γ = = = ρg V V C:\Adata\CLASNOTE\342\Class Notes\Key concepts_Topic 1.doc 1 γ Specific gravity is density normalized to the density of water: sg..= i γ w V 1 Specific volume is volume/mass: V = = m ρ Bulk modulus or modulus of elasticity is the pressure change per dp dp fractional change in volume or density: E =− = . -

1. Define Open, Closed, Or Isolated Systems. If You Use an Open System As a Calorimeter, What Is the State Function You Can Calculate from the Temperature Change
CH301 Worksheet 13b Answer Key—Internal Energy Lecture 1. Define open, closed, or isolated systems. If you use an open system as a calorimeter, what is the state function you can calculate from the temperature change. If you use a closed system as a calorimeter, what is the state function you can calculate from the temperature? Answer: An open system can exchange both matter and energy with the surroundings. Δ H is measured when an open system is used as a calorimeter. A closed system has a fixed amount of matter, but it can exchange energy with the surroundings. Δ U is measured when a closed system is used as a calorimeter because there is no change in volume and thus no expansion work can be done. An isolated system has no contact with its surroundings. The universe is considered an isolated system but on a less profound scale, your thermos for keeping liquids hot approximates an isolated system. 2. Rank, from greatest to least, the types internal energy found in a chemical system: Answer: The energy that holds the nucleus together is much greater than the energy in chemical bonds (covalent, metallic, network, ionic) which is much greater than IMF (Hydrogen bonding, dipole, London) which depending on temperature are approximate in value to motional energy (vibrational, rotational, translational). 3. Internal energy is a state function. Work and heat (w and q) are not. Explain. Answer: A state function (like U, V, T, S, G) depends only on the current state of the system so if the system is changed from one state to another, the change in a state function is independent of the path. -

Heat and Energy Conservation
1 Lecture notes in Fluid Dynamics (1.63J/2.01J) by Chiang C. Mei, MIT, Spring, 2007 CHAPTER 4. THERMAL EFFECTS IN FLUIDS 4-1-2energy.tex 4.1 Heat and energy conservation Recall the basic equations for a compressible fluid. Mass conservation requires that : ρt + ∇ · ρ~q = 0 (4.1.1) Momentum conservation requires that : = ρ (~qt + ~q∇ · ~q)= −∇p + ∇· τ +ρf~ (4.1.2) = where the viscous stress tensor τ has the components = ∂qi ∂qi ∂qk τ = τij = µ + + λ δij ij ∂xj ∂xi ! ∂xk There are 5 unknowns ρ, p, qi but only 4 equations. One more equation is needed. 4.1.1 Conservation of total energy Consider both mechanical ad thermal energy. Let e be the internal (thermal) energy per unit mass due to microscopic motion, and q2/2 be the kinetic energy per unit mass due to macroscopic motion. Conservation of energy requires D q2 ρ e + dV rate of incr. of energy in V (t) Dt ZZZV 2 ! = − Q~ · ~ndS rate of heat flux into V ZZS + ρf~ · ~qdV rate of work by body force ZZZV + Σ~ · ~qdS rate of work by surface force ZZX Use the kinematic transport theorm, the left hand side becomes D q2 ρ e + dV ZZZV Dt 2 ! 2 Using Gauss theorem the heat flux term becomes ∂Qi − QinidS = − dV ZZS ZZZV ∂xi The work done by surface stress becomes Σjqj dS = (σjini)qj dS ZZS ZZS ∂(σijqj) = (σijqj)ni dS = dV ZZS ZZZV ∂xi Now all terms are expressed as volume integrals over an arbitrary material volume, the following must be true at every point in space, 2 D q ∂Qi ∂(σijqi) ρ e + = − + ρfiqi + (4.1.3) Dt 2 ! ∂xi ∂xj As an alternative form, we differentiate the kinetic energy and get De -

Lecture 5: Magnetic Mirroring
!"#$%&'()%"*#%*+,-./-*+01.2(.*3+456789* !"#$%&"'()'*+,-".#'*/&&0&/-,' Dr. Peter T. Gallagher Astrophysics Research Group Trinity College Dublin :&2-;-)(*!"<-$2-"(=*%>*/-?"=)(*/%/="#* o Gyrating particle constitutes an electric current loop with a dipole moment: 1/2mv2 µ = " B o The dipole moment is conserved, i.e., is invariant. Called the first adiabatic invariant. ! o µ = constant even if B varies spatially or temporally. If B varies, then vperp varies to keep µ = constant => v|| also changes. o Gives rise to magnetic mirroring. Seen in planetary magnetospheres, magnetic bottles, coronal loops, etc. Bz o Right is geometry of mirror Br from Chen, Page 30. @-?"=)(*/2$$%$2"?* o Consider B-field pointed primarily in z-direction and whose magnitude varies in z- direction. If field is axisymmetric, B! = 0 and d/d! = 0. o This has cylindrical symmetry, so write B = Brrˆ + Bzzˆ o How does this configuration give rise to a force that can trap a charged particle? ! o Can obtain Br from " #B = 0 . In cylindrical polar coordinates: 1 " "Bz (rBr )+ = 0 r "r "z ! " "Bz => (rBr ) = #r "r "z o If " B z / " z is given at r = 0 and does not vary much with r, then r $Bz 1 2&$ Bz ) ! rBr = " #0 r dr % " r ( + $z 2 ' $z *r =0 ! 1 &$ Bz ) Br = " r( + (5.1) 2 ' $z *r =0 ! @-?"=)(*/2$$%$2"?* o Now have Br in terms of BZ, which we can use to find Lorentz force on particle. o The components of Lorentz force are: Fr = q(v" Bz # vzB" ) (1) F" = q(#vrBz + vzBr ) (2) (3) Fz = q(vrB" # v" Br ) (4) o As B! = 0, two terms vanish and terms (1) and (2) give rise to Larmor gyration. -

Chapter 15 - Fluid Mechanics Thursday, March 24Th
Chapter 15 - Fluid Mechanics Thursday, March 24th •Fluids – Static properties • Density and pressure • Hydrostatic equilibrium • Archimedes principle and buoyancy •Fluid Motion • The continuity equation • Bernoulli’s effect •Demonstration, iClicker and example problems Reading: pages 243 to 255 in text book (Chapter 15) Definitions: Density Pressure, ρ , is defined as force per unit area: Mass M ρ = = [Units – kg.m-3] Volume V Definition of mass – 1 kg is the mass of 1 liter (10-3 m3) of pure water. Therefore, density of water given by: Mass 1 kg 3 −3 ρH O = = 3 3 = 10 kg ⋅m 2 Volume 10− m Definitions: Pressure (p ) Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A Atmospheric pressure (1 atm.) is equal to 101325 N.m-2. 1 pound per square inch (1 psi) is equal to: 1 psi = 6944 Pa = 0.068 atm 1atm = 14.7 psi Definitions: Pressure (p ) Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A Pressure in Fluids Pressure, " p, is defined as force per unit area: # Force F p = = [Units – N.m-2, or Pascal (Pa)] " A8" rea A + $ In the presence of gravity, pressure in a static+ 8" fluid increases with depth. " – This allows an upward pressure force " to balance the downward gravitational force. + " $ – This condition is hydrostatic equilibrium. – Incompressible fluids like liquids have constant density; for them, pressure as a function of depth h is p p gh = 0+ρ p0 = pressure at surface " + Pressure in Fluids Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A In the presence of gravity, pressure in a static fluid increases with depth. -

Fluid Dynamics and Mantle Convection
Subduction II Fundamentals of Mantle Dynamics Thorsten W Becker University of Southern California Short course at Universita di Roma TRE April 18 – 20, 2011 Rheology Elasticity vs. viscous deformation η = O (1021) Pa s = viscosity µ= O (1011) Pa = shear modulus = rigidity τ = η / µ = O(1010) sec = O(103) years = Maxwell time Elastic deformation In general: σ ε ij = Cijkl kl (in 3-D 81 degrees of freedom, in general 21 independent) For isotropic body this reduces to Hooke’s law: σ λε δ µε ij = kk ij + 2 ij λ µ ε ε ε ε with and Lame’s parameters, kk = 11 + 22 + 33 Taking shear components ( i ≠ j ) gives definition of rigidity: σ µε 12 = 2 12 Adding the normal components ( i=j ) for all i=1,2,3 gives: σ λ µ ε κε kk = (3 + 2 ) kk = 3 kk with κ = λ + 2µ/3 = bulk modulus Linear viscous deformation (1) Total stress field = static + dynamic part: σ = − δ +τ ij p ij ij Analogous to elasticity … General case: τ = ε ij C'ijkl kl Isotropic case: τ = λ ε δ + ηε ij ' kk ij 2 ij Linear viscous deformation (2) Split in isotropic and deviatoric part (latter causes deformation): 1 σ ' = σ − σ δ = σ + pδ ij ij 3 kk ij ij ij 1 ε' = ε − ε δ ij ij 3 kk ij which gives the following stress: σ = − δ + ςε δ + ηε 'ij ( p p) ij kk ij 2 'ij ε = With compressibility term assumed 0 (Stokes condition kk 0 ) 2 ( ς = λ ' + η = bulk viscosity) 3 τ Now τ = 2 η ε or η = ij ij ij ε 2 ij η = In general f (T,d, p, H2O) Non-linear (or non-Newtonian) deformation General stress-strain relation: ε = Aτ n n = 1 : Newtonian n > 1 : non-Newtonian n → ∞ : pseudo-brittle 1 −1 1−n 1 −1/ n (1−n) / n Effective viscosity η = A τ = A ε eff 2 2 Application: different viscosities under oceans with different absolute plate motion, anisotropic viscosities by means of superposition (Schmeling, 1987) Microphysical theory and observations Maximum strength of materials (1) ‘Strength’ is maximum stress that material can resist In principle, viscous fluid has zero strength. -

First Law of Thermodynamics Control Mass (Closed System)
First Law of Thermodynamics Reading Problems 3-2 ! 3-7 3-40, 3-54, 3-105 5-1 ! 5-2 5-8, 5-25, 5-29, 5-37, 5-40, 5-42, 5-63, 5-74, 5-84, 5-109 6-1 ! 6-5 6-44, 6-51, 6-60, 6-80, 6-94, 6-124, 6-168, 6-173 Control Mass (Closed System) In this section we will examine the case of a control surface that is closed to mass flow, so that no mass can escape or enter the defined control region. Conservation of Mass Conservation of Mass, which states that mass cannot be created or destroyed, is implicitly satisfied by the definition of a control mass. Conservation of Energy The first law of thermodynamics states “Energy cannot be created or destroyed it can only change forms”. energy entering - energy leaving = change of energy within the system Sign Convention Cengel Approach Heat Transfer: heat transfer to a system is positive and heat transfer from a system is negative. Work Transfer: work done by a system is positive and work done on a system is negative. For instance: moving boundary work is defined as: Z 2 Wb = P dV 1 During a compression process, work is done on the system and the change in volume goes negative, i.e. dV < 0. In this case the boundary work will also be negative. 1 Culham Approach Using my sign convention, the boundary work is defined as: Z 2 Wb = − P dV 1 During a compression process, the change in volume is still negative but because of the negative sign on the right side of the boundary work equation, the bound- ary work directed into the system is considered positive. -

Experiment 7: Conservation of Energy
Experiment 7: Conservation of Energy One of the most important and useful concepts in mechanics is that of \Conservation of Energy". In this experiment, you will make measurements to demonstrate the conservation of mechanical energy and its transformation between kinetic energy and potential energy. The Total Mechanical Energy, E, of a system is defined as the sum of the Kinetic Energy, K, and the Potential Energy, U: E = K + U: (1) If the system is isolated, with only conservative forces acting on its parts, the total mechanical energy of the system is a constant. The mechanical energy can, however, be transformed between its kinetic and potential forms. cannot be destroyed. Rather, the energy is transmitted from one form to another. Any change in the kinetic energy will cause a corresponding change in the potential energy, and vice versa. The conservation of energy then dictates that, ∆K + ∆U = 0 (2) where ∆K is the change in the kinetic energy, and ∆U is the change in potential energy. Potential energy is a form of stored energy and is a consequence of the work done by a force. Examples of forces which have an associated potential energy are the gravitational and the electromagnetic fields and, in mechanics, a spring. In a sense potential energy is a storage system for energy. For a body moving under the influence of a force F , the change in potential energy is given by Z f −! −! ∆U = − F · ds (3) i where i and f represent the initial and final positions of the body, respectively. Hence, from Equation 2 we have what is commonly referred to as the work-kinetic energy theorem: Z f −! −! ∆K = F · ds (4) i Consider a body of mass m, being accelerated by a compressed spring.