Chapter 15 - Fluid Mechanics Thursday, March 24Th
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fluid Mechanics
FLUID MECHANICS PROF. DR. METİN GÜNER COMPILER ANKARA UNIVERSITY FACULTY OF AGRICULTURE DEPARTMENT OF AGRICULTURAL MACHINERY AND TECHNOLOGIES ENGINEERING 1 1. INTRODUCTION Mechanics is the oldest physical science that deals with both stationary and moving bodies under the influence of forces. Mechanics is divided into three groups: a) Mechanics of rigid bodies, b) Mechanics of deformable bodies, c) Fluid mechanics Fluid mechanics deals with the behavior of fluids at rest (fluid statics) or in motion (fluid dynamics), and the interaction of fluids with solids or other fluids at the boundaries (Fig.1.1.). Fluid mechanics is the branch of physics which involves the study of fluids (liquids, gases, and plasmas) and the forces on them. Fluid mechanics can be divided into two. a)Fluid Statics b)Fluid Dynamics Fluid statics or hydrostatics is the branch of fluid mechanics that studies fluids at rest. It embraces the study of the conditions under which fluids are at rest in stable equilibrium Hydrostatics is fundamental to hydraulics, the engineering of equipment for storing, transporting and using fluids. Hydrostatics offers physical explanations for many phenomena of everyday life, such as why atmospheric pressure changes with altitude, why wood and oil float on water, and why the surface of water is always flat and horizontal whatever the shape of its container. Fluid dynamics is a subdiscipline of fluid mechanics that deals with fluid flow— the natural science of fluids (liquids and gases) in motion. It has several subdisciplines itself, including aerodynamics (the study of air and other gases in motion) and hydrodynamics (the study of liquids in motion). -

Fluid Mechanics
cen72367_fm.qxd 11/23/04 11:22 AM Page i FLUID MECHANICS FUNDAMENTALS AND APPLICATIONS cen72367_fm.qxd 11/23/04 11:22 AM Page ii McGRAW-HILL SERIES IN MECHANICAL ENGINEERING Alciatore and Histand: Introduction to Mechatronics and Measurement Systems Anderson: Computational Fluid Dynamics: The Basics with Applications Anderson: Fundamentals of Aerodynamics Anderson: Introduction to Flight Anderson: Modern Compressible Flow Barber: Intermediate Mechanics of Materials Beer/Johnston: Vector Mechanics for Engineers Beer/Johnston/DeWolf: Mechanics of Materials Borman and Ragland: Combustion Engineering Budynas: Advanced Strength and Applied Stress Analysis Çengel and Boles: Thermodynamics: An Engineering Approach Çengel and Cimbala: Fluid Mechanics: Fundamentals and Applications Çengel and Turner: Fundamentals of Thermal-Fluid Sciences Çengel: Heat Transfer: A Practical Approach Crespo da Silva: Intermediate Dynamics Dieter: Engineering Design: A Materials & Processing Approach Dieter: Mechanical Metallurgy Doebelin: Measurement Systems: Application & Design Dunn: Measurement & Data Analysis for Engineering & Science EDS, Inc.: I-DEAS Student Guide Hamrock/Jacobson/Schmid: Fundamentals of Machine Elements Henkel and Pense: Structure and Properties of Engineering Material Heywood: Internal Combustion Engine Fundamentals Holman: Experimental Methods for Engineers Holman: Heat Transfer Hsu: MEMS & Microsystems: Manufacture & Design Hutton: Fundamentals of Finite Element Analysis Kays/Crawford/Weigand: Convective Heat and Mass Transfer Kelly: Fundamentals -
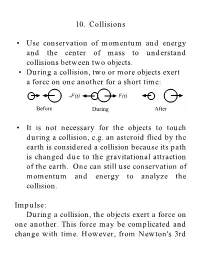
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Equation of State for the Lennard-Jones Fluid
Equation of State for the Lennard-Jones Fluid Monika Thol1*, Gabor Rutkai2, Andreas Köster2, Rolf Lustig3, Roland Span1, Jadran Vrabec2 1Lehrstuhl für Thermodynamik, Ruhr-Universität Bochum, Universitätsstraße 150, 44801 Bochum, Germany 2Lehrstuhl für Thermodynamik und Energietechnik, Universität Paderborn, Warburger Straße 100, 33098 Paderborn, Germany 3Department of Chemical and Biomedical Engineering, Cleveland State University, Cleveland, Ohio 44115, USA Abstract An empirical equation of state correlation is proposed for the Lennard-Jones model fluid. The equation in terms of the Helmholtz energy is based on a large molecular simulation data set and thermal virial coefficients. The underlying data set consists of directly simulated residual Helmholtz energy derivatives with respect to temperature and density in the canonical ensemble. Using these data introduces a new methodology for developing equations of state from molecular simulation data. The correlation is valid for temperatures 0.5 < T/Tc < 7 and pressures up to p/pc = 500. Extensive comparisons to simulation data from the literature are made. The accuracy and extrapolation behavior is better than for existing equations of state. Key words: equation of state, Helmholtz energy, Lennard-Jones model fluid, molecular simulation, thermodynamic properties _____________ *E-mail: [email protected] Content Content ....................................................................................................................................... 2 List of Tables ............................................................................................................................. -

Estimating Your Air Consumption
10/29/2019 Alert Diver | Estimating Your Air Consumption Estimating Your Air Consumption Advanced Diving Public Safety Diving By Mike Ange Mastering Neutral Buoyancy and Trim Military Diving Technical Diving Scientific Diving and Safety Program Oversight Seeing the Reef in a New Light ADVERTISEMENT Do you have enough breathing gas to complete the next dive? Here's how to find out. It is a warm clear day, and the Atlantic Ocean is like glass. As you drop into the water for a dive on North Carolina's famous U-352 wreck, you can see that the :: captain has hooked the wreck very near the stern. It is your plan to circumnavigate the entire structure and get that perfect photograph near the exposed bow torpedo tube. You descend to slightly below 100 feet, reach the structure and take off toward the bow. Unfortunately, you are only halfway, just approaching the conning tower, when your buddy signals that he is running low on air. Putting safety first, you return with him to the ascent line — cursing the lost opportunity and vowing to find a new buddy. If you've ever experienced the disappointment of ending a dive too soon for lack of breathing gas or, worse, had to make a hurried ascent because you ran out of air, it may surprise you to learn that your predicament was entirely predictable. With a little planning and some basic calculations, you can estimate how much breathing gas you will need to complete a dive and then take steps to ensure an adequate supply. It's a process that technical divers live by and one that can also be applied to basic open-water diving. -

Modeling Conservation of Mass
Modeling Conservation of Mass How is mass conserved (protected from loss)? Imagine an evening campfire. As the wood burns, you notice that the logs have become a small pile of ashes. What happened? Was the wood destroyed by the fire? A scientific principle called the law of conservation of mass states that matter is neither created nor destroyed. So, what happened to the wood? Think back. Did you observe smoke rising from the fire? When wood burns, atoms in the wood combine with oxygen atoms in the air in a chemical reaction called combustion. The products of this burning reaction are ashes as well as the carbon dioxide and water vapor in smoke. The gases escape into the air. We also know from the law of conservation of mass that the mass of the reactants must equal the mass of all the products. How does that work with the campfire? mass – a measure of how much matter is present in a substance law of conservation of mass – states that the mass of all reactants must equal the mass of all products and that matter is neither created nor destroyed If you could measure the mass of the wood and oxygen before you started the fire and then measure the mass of the smoke and ashes after it burned, what would you find? The total mass of matter after the fire would be the same as the total mass of matter before the fire. Therefore, matter was neither created nor destroyed in the campfire; it just changed form. The same atoms that made up the materials before the reaction were simply rearranged to form the materials left after the reaction. -

1 Fluid Flow Outline Fundamentals of Rheology
Fluid Flow Outline • Fundamentals and applications of rheology • Shear stress and shear rate • Viscosity and types of viscometers • Rheological classification of fluids • Apparent viscosity • Effect of temperature on viscosity • Reynolds number and types of flow • Flow in a pipe • Volumetric and mass flow rate • Friction factor (in straight pipe), friction coefficient (for fittings, expansion, contraction), pressure drop, energy loss • Pumping requirements (overcoming friction, potential energy, kinetic energy, pressure energy differences) 2 Fundamentals of Rheology • Rheology is the science of deformation and flow – The forces involved could be tensile, compressive, shear or bulk (uniform external pressure) • Food rheology is the material science of food – This can involve fluid or semi-solid foods • A rheometer is used to determine rheological properties (how a material flows under different conditions) – Viscometers are a sub-set of rheometers 3 1 Applications of Rheology • Process engineering calculations – Pumping requirements, extrusion, mixing, heat transfer, homogenization, spray coating • Determination of ingredient functionality – Consistency, stickiness etc. • Quality control of ingredients or final product – By measurement of viscosity, compressive strength etc. • Determination of shelf life – By determining changes in texture • Correlations to sensory tests – Mouthfeel 4 Stress and Strain • Stress: Force per unit area (Units: N/m2 or Pa) • Strain: (Change in dimension)/(Original dimension) (Units: None) • Strain rate: Rate -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

8. Decompression Procedures Diver
TDI Standards and Procedures Part 2: TDI Diver Standards 8. Decompression Procedures Diver 8.1 Introduction This course examines the theory, methods and procedures of planned stage decompression diving. This program is designed as a stand-alone course or it may be taught in conjunction with TDI Advanced Nitrox, Advanced Wreck, or Full Cave Course. The objective of this course is to train divers how to plan and conduct a standard staged decompression dive not exceeding a maximum depth of 45 metres / 150 feet. The most common equipment requirements, equipment set-up and decompression techniques are presented. Students are permitted to utilize enriched air nitrox (EAN) mixes or oxygen for decompression provided the gas mix is within their current certification level. 8.2 Qualifications of Graduates Upon successful completion of this course, graduates may engage in decompression diving activities without direct supervision provided: 1. The diving activities approximate those of training 2. The areas of activities approximate those of training 3. Environmental conditions approximate those of training Upon successful completion of this course, graduates are qualified to enroll in: 1. TDI Advanced Nitrox Course 2. TDI Extended Range Course 3. TDI Advanced Wreck Course 4. TDI Trimix Course 8.3 Who May Teach Any active TDI Decompression Procedures Instructor may teach this course Version 0221 67 TDI Standards and Procedures Part 2: TDI Diver Standards 8.4 Student to Instructor Ratio Academic 1. Unlimited, so long as adequate facility, supplies and time are provided to ensure comprehensive and complete training of subject matter Confined Water (swimming pool-like conditions) 1. -

Chapter 3 Equations of State
Chapter 3 Equations of State The simplest way to derive the Helmholtz function of a fluid is to directly integrate the equation of state with respect to volume (Sadus, 1992a, 1994). An equation of state can be applied to either vapour-liquid or supercritical phenomena without any conceptual difficulties. Therefore, in addition to liquid-liquid and vapour -liquid properties, it is also possible to determine transitions between these phenomena from the same inputs. All of the physical properties of the fluid except ideal gas are also simultaneously calculated. Many equations of state have been proposed in the literature with either an empirical, semi- empirical or theoretical basis. Comprehensive reviews can be found in the works of Martin (1979), Gubbins (1983), Anderko (1990), Sandler (1994), Economou and Donohue (1996), Wei and Sadus (2000) and Sengers et al. (2000). The van der Waals equation of state (1873) was the first equation to predict vapour-liquid coexistence. Later, the Redlich-Kwong equation of state (Redlich and Kwong, 1949) improved the accuracy of the van der Waals equation by proposing a temperature dependence for the attractive term. Soave (1972) and Peng and Robinson (1976) proposed additional modifications of the Redlich-Kwong equation to more accurately predict the vapour pressure, liquid density, and equilibria ratios. Guggenheim (1965) and Carnahan and Starling (1969) modified the repulsive term of van der Waals equation of state and obtained more accurate expressions for hard sphere systems. Christoforakos and Franck (1986) modified both the attractive and repulsive terms of van der Waals equation of state. Boublik (1981) extended the Carnahan-Starling hard sphere term to obtain an accurate equation for hard convex geometries. -

Two-Way Fluid–Solid Interaction Analysis for a Horizontal Axis Marine Current Turbine with LES
water Article Two-Way Fluid–Solid Interaction Analysis for a Horizontal Axis Marine Current Turbine with LES Jintong Gu 1,2, Fulin Cai 1,*, Norbert Müller 2, Yuquan Zhang 3 and Huixiang Chen 2,4 1 College of Water Conservancy and Hydropower Engineering, Hohai University, Nanjing 210098, China; [email protected] 2 Department of Mechanical Engineering, Michigan State University, East Lansing, MI 48824, USA; [email protected] (N.M.); [email protected] (H.C.) 3 College of Energy and Electrical Engineering, Hohai University, Nanjing 210098, China; [email protected] 4 College of Agricultural Engineering, Hohai University, Nanjing 210098, China * Correspondence: fl[email protected]; Tel.: +86-139-5195-1792 Received: 2 September 2019; Accepted: 23 December 2019; Published: 27 December 2019 Abstract: Operating in the harsh marine environment, fluctuating loads due to the surrounding turbulence are important for fatigue analysis of marine current turbines (MCTs). The large eddy simulation (LES) method was implemented to analyze the two-way fluid–solid interaction (FSI) for an MCT. The objective was to afford insights into the hydrodynamics near the rotor and in the wake, the deformation of rotor blades, and the interaction between the solid and fluid field. The numerical fluid simulation results showed good agreement with the experimental data and the influence of the support on the power coefficient and blade vibration. The impact of the blade displacement on the MCT performance was quantitatively analyzed. Besides the root, the highest stress was located near the middle of the blade. The findings can inform the design of MCTs for enhancing robustness and survivability. -

Lecture 1: Introduction
Lecture 1: Introduction E. J. Hinch Non-Newtonian fluids occur commonly in our world. These fluids, such as toothpaste, saliva, oils, mud and lava, exhibit a number of behaviors that are different from Newtonian fluids and have a number of additional material properties. In general, these differences arise because the fluid has a microstructure that influences the flow. In section 2, we will present a collection of some of the interesting phenomena arising from flow nonlinearities, the inhibition of stretching, elastic effects and normal stresses. In section 3 we will discuss a variety of devices for measuring material properties, a process known as rheometry. 1 Fluid Mechanical Preliminaries The equations of motion for an incompressible fluid of unit density are (for details and derivation see any text on fluid mechanics, e.g. [1]) @u + (u · r) u = r · S + F (1) @t r · u = 0 (2) where u is the velocity, S is the total stress tensor and F are the body forces. It is customary to divide the total stress into an isotropic part and a deviatoric part as in S = −pI + σ (3) where tr σ = 0. These equations are closed only if we can relate the deviatoric stress to the velocity field (the pressure field satisfies the incompressibility condition). It is common to look for local models where the stress depends only on the local gradients of the flow: σ = σ (E) where E is the rate of strain tensor 1 E = ru + ruT ; (4) 2 the symmetric part of the the velocity gradient tensor. The trace-free requirement on σ and the physical requirement of symmetry σ = σT means that there are only 5 independent components of the deviatoric stress: 3 shear stresses (the off-diagonal elements) and 2 normal stress differences (the diagonal elements constrained to sum to 0).