Heat and Energy Conservation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
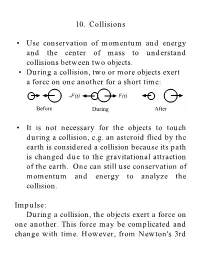
10. Collisions • Use Conservation of Momentum and Energy and The
10. Collisions • Use conservation of momentum and energy and the center of mass to understand collisions between two objects. • During a collision, two or more objects exert a force on one another for a short time: -F(t) F(t) Before During After • It is not necessary for the objects to touch during a collision, e.g. an asteroid flied by the earth is considered a collision because its path is changed due to the gravitational attraction of the earth. One can still use conservation of momentum and energy to analyze the collision. Impulse: During a collision, the objects exert a force on one another. This force may be complicated and change with time. However, from Newton's 3rd Law, the two objects must exert an equal and opposite force on one another. F(t) t ti tf Dt From Newton'sr 2nd Law: dp r = F (t) dt r r dp = F (t)dt r r r r tf p f - pi = Dp = ò F (t)dt ti The change in the momentum is defined as the impulse of the collision. • Impulse is a vector quantity. Impulse-Linear Momentum Theorem: In a collision, the impulse on an object is equal to the change in momentum: r r J = Dp Conservation of Linear Momentum: In a system of two or more particles that are colliding, the forces that these objects exert on one another are internal forces. These internal forces cannot change the momentum of the system. Only an external force can change the momentum. The linear momentum of a closed isolated system is conserved during a collision of objects within the system. -

Law of Conversation of Energy
Law of Conservation of Mass: "In any kind of physical or chemical process, mass is neither created nor destroyed - the mass before the process equals the mass after the process." - the total mass of the system does not change, the total mass of the products of a chemical reaction is always the same as the total mass of the original materials. "Physics for scientists and engineers," 4th edition, Vol.1, Raymond A. Serway, Saunders College Publishing, 1996. Ex. 1) When wood burns, mass seems to disappear because some of the products of reaction are gases; if the mass of the original wood is added to the mass of the oxygen that combined with it and if the mass of the resulting ash is added to the mass o the gaseous products, the two sums will turn out exactly equal. 2) Iron increases in weight on rusting because it combines with gases from the air, and the increase in weight is exactly equal to the weight of gas consumed. Out of thousands of reactions that have been tested with accurate chemical balances, no deviation from the law has ever been found. Law of Conversation of Energy: The total energy of a closed system is constant. Matter is neither created nor destroyed – total mass of reactants equals total mass of products You can calculate the change of temp by simply understanding that energy and the mass is conserved - it means that we added the two heat quantities together we can calculate the change of temperature by using the law or measure change of temp and show the conservation of energy E1 + E2 = E3 -> E(universe) = E(System) + E(Surroundings) M1 + M2 = M3 Is T1 + T2 = unknown (No, no law of conservation of temperature, so we have to use the concept of conservation of energy) Total amount of thermal energy in beaker of water in absolute terms as opposed to differential terms (reference point is 0 degrees Kelvin) Knowns: M1, M2, T1, T2 (Kelvin) When add the two together, want to know what T3 and M3 are going to be. -

Energy Conservation
2016 Centre County Planning Opportunities Energy Conservation Centre County Comprehensive Plan — Phase II Implementation Strategies Introduction County-wide In 2003, the Centre County Board of Commissioners Planning adopted a County-wide Comprehensive Plan which included Goals background studies, inventories of existing conditions, goals and recommendations. These recommendations, revised Adopted 2003 and updated, continue to serve as a vision and a general direction for policy and community improvement. Those specific to energy conservation will be discussed here along with implementation strategies to achieve the recom- #1 — Identify, pre- mendations. For more detailed background information serve, enhance and monitor agricultural please refer to the 2003 Comprehensive Plan available on resources. the Centre County Planning and Community Development webpage: #2 — Identify, pre- serve, and monitor http://centrecountypa.gov/index.aspx?nid=212. environmental and Centre County seeks to balance growth, protection of natural resources. resources, investment in compatible new building Small wind turbines like erected #3 — Preserve his- development, and incentives for sustainable development. at the DEP Moshannon Office, toric and cultural Much of this effort includes stewardship, community can help offset electricity costs resources. outreach and expert professional service. to the property. #4 — Ensure decent, safe, sanitary and affordable housing in suitable living surroundings, com- patible with the en- vironment for all The Keystone Principles individuals. In 2005, Pennsylvania adopt- Redevelop first #5 — Appropriately ed the “Keystone Principles Provide efficient infrastructure locate and maintain for Growth, Investment and existing and pro- Resource Conservation”, a Concentrate development posed community set of principles that have Increase job opportunities facilities, utilities, focused Pennsylvania on and services for all Foster sustainable businesses reinvestment and reuse of its residents. -

Adaptive Equipment and Energy Conservation Techniques During Performance of Activities of Daily Living
Adaptive Equipment and Energy Conservation Techniques During Performance of Activities of Daily Living Problem: A wide range of diagnosis can affect the performance of activities of daily living (ADLs). The performance of these activities; feeding, dressing, and bathing to name a few are an essential part of our daily lives. An individual’s ability to function in daily activities is often dependent on physical and cognitive health. The use of adaptive equipment and energy conservation techniques can make all the difference in making these important daily tasks possible and effect one’s perception and quality of their life. Adaptive Equipment Adaptive equipment is used to improve functional capabilities. Adaptations can assist someone in their home or out in the community, ranging from longer, thicker handles on brushes and silver wear for making them easier to grasp to a powered wheelchair. Below is a chart including various diagnosis and examples of adaptive equipment that could greatly benefit individuals experiencing similar circumstances. The equipment listed will promote functional independence as well as safety during performance of ADLs. Diagnosis Adaptive Rationale for Equipment Price Website/Resourc Equipment Range e Link to Purchase Equipment Joint Reacher This assistive device can help in $5.50 - https://www.healthpro Replacement accessing spaces that may be $330.00 ductsforyou.com/p- hard for the individual to reach featherlite- (THA/TKR) otherwise. Frequent sitting and reacher.html standing (bending more than 90 degrees) are not recommended for individuals with a recent joint replacement. This tool will allow the individual to grasp an object further away without movement of lower extremities. -

Improving Institutional Access to Financing Incentives for Energy
Improving Institutional Access to Financing Incentives for Energy Demand Reductions Masters Project: Final Report April 2016 Sponsor Agency: The Ecology Center (Ann Arbor, MI) Student Team: Brian La Shier, Junhong Liang, Chayatach Pasawongse, Gianna Petito, & Whitney Smith Faculty Advisors: Paul Mohai PhD. & Tony Reames PhD. ACKNOWLEDGEMENTS We would like to thank our clients Alexis Blizman and Katy Adams from the Ecology Center. We greatly appreciate their initial efforts in conceptualizing and proposing the project idea, and providing feedback throughout the duration of the project. We would also like to thank our advisors Dr. Paul Mohai and Dr. Tony Reames for providing their expertise, guidance, and support. This Master's Project report submitted in partial fulfillment of the OPUS requirements for the degree of Master of Science, Natural Resources and Environment, University of Michigan. ABSTRACT We developed this project in response to a growing locallevel demand for information and guidance on accessing local, state, and federal energy financing programs. Knowledge regarding these programs is currently scattered across independent websites and agencies, making it difficult for a lay user to identify available options for funding energy efficiency efforts. We collaborated with The Ecology Center, an Ann Arbor nonprofit, to develop an informationbased tool that would provide tailored recommendations to small businesses and organizations in need of financing to meet their energy efficiency aspirations. The tool was developed for use by The Ecology Center along with an implementation plan to strengthen their outreach to local stakeholders and assist their efforts in reducing Michigan’s energy consumption. We researched and analyzed existing clean energy and energy efficiency policies and financing opportunities available from local, state, federal, and utility entities for institutions in the educational, medical, religious, and multifamily housing sectors. -

Lecture 5: Magnetic Mirroring
!"#$%&'()%"*#%*+,-./-*+01.2(.*3+456789* !"#$%&"'()'*+,-".#'*/&&0&/-,' Dr. Peter T. Gallagher Astrophysics Research Group Trinity College Dublin :&2-;-)(*!"<-$2-"(=*%>*/-?"=)(*/%/="#* o Gyrating particle constitutes an electric current loop with a dipole moment: 1/2mv2 µ = " B o The dipole moment is conserved, i.e., is invariant. Called the first adiabatic invariant. ! o µ = constant even if B varies spatially or temporally. If B varies, then vperp varies to keep µ = constant => v|| also changes. o Gives rise to magnetic mirroring. Seen in planetary magnetospheres, magnetic bottles, coronal loops, etc. Bz o Right is geometry of mirror Br from Chen, Page 30. @-?"=)(*/2$$%$2"?* o Consider B-field pointed primarily in z-direction and whose magnitude varies in z- direction. If field is axisymmetric, B! = 0 and d/d! = 0. o This has cylindrical symmetry, so write B = Brrˆ + Bzzˆ o How does this configuration give rise to a force that can trap a charged particle? ! o Can obtain Br from " #B = 0 . In cylindrical polar coordinates: 1 " "Bz (rBr )+ = 0 r "r "z ! " "Bz => (rBr ) = #r "r "z o If " B z / " z is given at r = 0 and does not vary much with r, then r $Bz 1 2&$ Bz ) ! rBr = " #0 r dr % " r ( + $z 2 ' $z *r =0 ! 1 &$ Bz ) Br = " r( + (5.1) 2 ' $z *r =0 ! @-?"=)(*/2$$%$2"?* o Now have Br in terms of BZ, which we can use to find Lorentz force on particle. o The components of Lorentz force are: Fr = q(v" Bz # vzB" ) (1) F" = q(#vrBz + vzBr ) (2) (3) Fz = q(vrB" # v" Br ) (4) o As B! = 0, two terms vanish and terms (1) and (2) give rise to Larmor gyration. -

Chapter 15 - Fluid Mechanics Thursday, March 24Th
Chapter 15 - Fluid Mechanics Thursday, March 24th •Fluids – Static properties • Density and pressure • Hydrostatic equilibrium • Archimedes principle and buoyancy •Fluid Motion • The continuity equation • Bernoulli’s effect •Demonstration, iClicker and example problems Reading: pages 243 to 255 in text book (Chapter 15) Definitions: Density Pressure, ρ , is defined as force per unit area: Mass M ρ = = [Units – kg.m-3] Volume V Definition of mass – 1 kg is the mass of 1 liter (10-3 m3) of pure water. Therefore, density of water given by: Mass 1 kg 3 −3 ρH O = = 3 3 = 10 kg ⋅m 2 Volume 10− m Definitions: Pressure (p ) Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A Atmospheric pressure (1 atm.) is equal to 101325 N.m-2. 1 pound per square inch (1 psi) is equal to: 1 psi = 6944 Pa = 0.068 atm 1atm = 14.7 psi Definitions: Pressure (p ) Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A Pressure in Fluids Pressure, " p, is defined as force per unit area: # Force F p = = [Units – N.m-2, or Pascal (Pa)] " A8" rea A + $ In the presence of gravity, pressure in a static+ 8" fluid increases with depth. " – This allows an upward pressure force " to balance the downward gravitational force. + " $ – This condition is hydrostatic equilibrium. – Incompressible fluids like liquids have constant density; for them, pressure as a function of depth h is p p gh = 0+ρ p0 = pressure at surface " + Pressure in Fluids Pressure, p, is defined as force per unit area: Force F p = = [Units – N.m-2, or Pascal (Pa)] Area A In the presence of gravity, pressure in a static fluid increases with depth. -

Fluid Dynamics and Mantle Convection
Subduction II Fundamentals of Mantle Dynamics Thorsten W Becker University of Southern California Short course at Universita di Roma TRE April 18 – 20, 2011 Rheology Elasticity vs. viscous deformation η = O (1021) Pa s = viscosity µ= O (1011) Pa = shear modulus = rigidity τ = η / µ = O(1010) sec = O(103) years = Maxwell time Elastic deformation In general: σ ε ij = Cijkl kl (in 3-D 81 degrees of freedom, in general 21 independent) For isotropic body this reduces to Hooke’s law: σ λε δ µε ij = kk ij + 2 ij λ µ ε ε ε ε with and Lame’s parameters, kk = 11 + 22 + 33 Taking shear components ( i ≠ j ) gives definition of rigidity: σ µε 12 = 2 12 Adding the normal components ( i=j ) for all i=1,2,3 gives: σ λ µ ε κε kk = (3 + 2 ) kk = 3 kk with κ = λ + 2µ/3 = bulk modulus Linear viscous deformation (1) Total stress field = static + dynamic part: σ = − δ +τ ij p ij ij Analogous to elasticity … General case: τ = ε ij C'ijkl kl Isotropic case: τ = λ ε δ + ηε ij ' kk ij 2 ij Linear viscous deformation (2) Split in isotropic and deviatoric part (latter causes deformation): 1 σ ' = σ − σ δ = σ + pδ ij ij 3 kk ij ij ij 1 ε' = ε − ε δ ij ij 3 kk ij which gives the following stress: σ = − δ + ςε δ + ηε 'ij ( p p) ij kk ij 2 'ij ε = With compressibility term assumed 0 (Stokes condition kk 0 ) 2 ( ς = λ ' + η = bulk viscosity) 3 τ Now τ = 2 η ε or η = ij ij ij ε 2 ij η = In general f (T,d, p, H2O) Non-linear (or non-Newtonian) deformation General stress-strain relation: ε = Aτ n n = 1 : Newtonian n > 1 : non-Newtonian n → ∞ : pseudo-brittle 1 −1 1−n 1 −1/ n (1−n) / n Effective viscosity η = A τ = A ε eff 2 2 Application: different viscosities under oceans with different absolute plate motion, anisotropic viscosities by means of superposition (Schmeling, 1987) Microphysical theory and observations Maximum strength of materials (1) ‘Strength’ is maximum stress that material can resist In principle, viscous fluid has zero strength. -

Energy Conservation Action Plan
2/2/12 Energy Conservation Action Plan Energy conservation is undertaken for a variety of reasons which includes utility cost containment and reduction of the carbon footprint. Incumbent upon all of us is the preservation of resources to perpetuate a quality life style. A holistic approach to conservation is articulated in this plan which outlines action items for an energy conservation program. This energy conservation plan is offered to discuss steps taken, work practices in place, new strategies, and energy conservation policies. At Creighton University, as is the case for most colleges and universities, it is recognized that deferred maintenance on buildings exists and as such, so does the inefficiency of operation. Advancing programs that reduce deferred maintenance will not be specifically addressed in this plan. A variety of action items to enhance energy conservation are offered in this plan to draw attention to a variety of tasks and opportunities that can be pursued. Action Item: A) Implement an Energy Conservation Policy: An energy conservation policy is needed to document the goals of the University in establishing recognition of energy savings. The energy conservation policy includes: Creating guidelines for proper management of our energy resources; (e.g. water, natural gas, and the energy products of steam, chilled water, and electricity). Controlling the waste of natural resources. Maintaining the most comfortable and safest environmental conditions in university buildings at the lowest cost. Creating an outline to be used for educating faculty, staff, students and guests of the University in the day to day practice of energy conservation. An updated but unapproved policy is attached for further discussion and consideration. -

Experiment 7: Conservation of Energy
Experiment 7: Conservation of Energy One of the most important and useful concepts in mechanics is that of \Conservation of Energy". In this experiment, you will make measurements to demonstrate the conservation of mechanical energy and its transformation between kinetic energy and potential energy. The Total Mechanical Energy, E, of a system is defined as the sum of the Kinetic Energy, K, and the Potential Energy, U: E = K + U: (1) If the system is isolated, with only conservative forces acting on its parts, the total mechanical energy of the system is a constant. The mechanical energy can, however, be transformed between its kinetic and potential forms. cannot be destroyed. Rather, the energy is transmitted from one form to another. Any change in the kinetic energy will cause a corresponding change in the potential energy, and vice versa. The conservation of energy then dictates that, ∆K + ∆U = 0 (2) where ∆K is the change in the kinetic energy, and ∆U is the change in potential energy. Potential energy is a form of stored energy and is a consequence of the work done by a force. Examples of forces which have an associated potential energy are the gravitational and the electromagnetic fields and, in mechanics, a spring. In a sense potential energy is a storage system for energy. For a body moving under the influence of a force F , the change in potential energy is given by Z f −! −! ∆U = − F · ds (3) i where i and f represent the initial and final positions of the body, respectively. Hence, from Equation 2 we have what is commonly referred to as the work-kinetic energy theorem: Z f −! −! ∆K = F · ds (4) i Consider a body of mass m, being accelerated by a compressed spring. -

Conservation of Momentum
AccessScience from McGraw-Hill Education Page 1 of 3 www.accessscience.com Conservation of momentum Contributed by: Paul W. Schmidt Publication year: 2014 The principle that, when a system of masses is subject only to forces that masses of the system exert on one another, the total vector momentum of the system is constant. Since vector momentum is conserved, in problems involving more than one dimension the component of momentum in any direction will remain constant. The principle of conservation of momentum holds generally and is applicable in all fields of physics. In particular, momentum is conserved even if the particles of a system exert forces on one another or if the total mechanical energy is not conserved. Use of the principle of conservation of momentum is fundamental in the solution of collision problems. See also: COLLISION (PHYSICS) . If a person standing on a well-lubricated cart steps forward, the cart moves backward. One can explain this result by momentum conservation, considering the system to consist of cart and human. If both person and cart are originally at rest, the momentum of the system is zero. If the person then acquires forward momentum by stepping forward, the cart must receive a backward momentum of equal magnitude in order for the system to retain a total momentum of zero. When the principle of conservation of momentum is applied, care must be taken that the system under consideration is really isolated. For example, when a rough rock rolls down a hill, the isolated system would have to consist of the rock plus the earth, and not the rock alone, since momentum exchanges between the rock and the earth cannot be neglected. -

Energy, Energy Conservation and the ICE Fund Tax Provincial Sales Tax Act
Provincial Sales Tax (PST) Bulletin Bulletin PST 203 Issued: March 2013 Revised: April 2019 Energy, Energy Conservation and the ICE Fund Tax Provincial Sales Tax Act Latest Revision: The revision bar ( ) identifies changes to the previous version of this bulletin dated November 2017. For a summary of the changes, see Latest Revision at the end of this document. This bulletin provides information on: . How PST applies to: • energy purchased in BC or brought, sent or delivered into BC • materials and equipment used to conserve energy . Exemptions for certain uses of energy . The 0.4% tax on energy products to raise revenue for the Innovative Clean Energy (ICE) Fund (ICE Fund tax) Note: Fuels used to power an internal combustion engine and propane for any use are not subject to PST but are taxed under the Motor Fuel Tax Act. For more information on motor fuel tax, see our Motor Fuel Tax and Carbon Tax page and Natural Gas below. Table of Contents Overview……………………………………………………………2 Definitions ................................................................................ 2 Residential Energy Products ................................................... 3 Qualifying Farmers .................................................................. 5 Electricity ................................................................................. 5 Exempt Fuels for Use as a Source of Energy .......................... 6 Goods Provided on a Continuous Basis Under Certain Contracts ................................................................... 6 Natural