Thermodynamic Analysis of an Irreversible Maisotsenko Reciprocating Brayton Cycle
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Analysis of the Brayton Cycle As a Cryogenic Refrigerator
jbrary, E-01 Admin. BJdg. OCT 7 1968 NBS 366 An Analysis of the Brayton Cycle As a Cryogenic Refrigerator ITATIONAL BURSAL' OF iTANDABD8 LIBRABT MAR 6 J973 V* 0F c J* 0a <5 Q \ v S. DEPARTMENT OF COMMERCE in Q National Bureau of Standards %. *^CAU 0? * : NATIONAL BUREAU OF STANDARDS The National Bureau of Standards 1 was established by an act of Congress March 3, 1901. Today, in addition to serving as the Nation's central measurement laboratory, the Bureau is a principal focal point in the Federal Government for assuring maxi- mum application of the physical and engineering sciences to the advancement of tech- nology in industry and commerce. To this end the Bureau conducts research and provides central national services in three broad program areas and provides cen- tral national services in a fourth. These are: (1) basic measurements and standards, (2) materials measurements and standards, (3) technological measurements and standards, and (4) transfer of technology. The Bureau comprises the Institute for Basic Standards, the Institute for Materials Research, the Institute for Applied Technology, and the Center for Radiation Research. THE INSTITUTE FOR BASIC STANDARDS provides the central basis within the United States of a complete and consistent system of physical measurement, coor- dinates that system with the measurement systems of other nations, and furnishes essential services leading to accurate and uniform physical measurements throughout the Nation's scientific community, industry, and commerce. The Institute consists of an Office of Standard Reference Data and a group of divisions organized by the following areas of science and engineering Applied Mathematics—Electricity—Metrology—Mechanics—Heat—Atomic Phys- 2 2 ics—Cryogenics-—Radio Physics-—Radio Engineering —Astrophysics —Time and Frequency. -
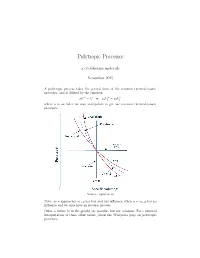
Polytropic Processes
Polytropic Processes a.cyclohexane.molecule November 2015 A polytropic process takes the general form of the common thermodynamic processes, and is defined by the equation n n n pV = C or p1V1 = p2V2 where n is an index we may manipulate to get our common thermodynamic processes. Source: nptel.ac.in Note: as n approaches 1, p has less and less influence; when n = 1, p has no influence and we thus have an isobaric process. Other n values (x in the graph) are possible, but not common. For a physical interpretation of these other values, check the Wikipedia page on polytropic processes. Because polytropic processes are general representations of thermodynamic pro- cesses, any quantities we calculate for a polytropic process will be a general equation for that quantity in all other thermodynamic processes. We proceed to find the work and heat associated with a polytropic process. We start with the definition of work: Z V2 w = − p dV V1 The polytropic equation pV n = C allows us to manipulate our formula for work in the following manner, as long as n 6= 1 V V Z 2 C Z 2 C V C V w = − dV = −C V −n dV = − V 1−n 2 = V 1−n 2 n V1 V1 V1 V V1 1 − n n − 1 Evaluating this expression, C CV 1−n − CV 1−n w = V 1−n − V 1−n = 2 1 n − 1 2 1 n − 1 n n We let the first C be p2V2 and the second C be p1V1 , such that the exponents cancel to obtain p V nV 1−n − p V nV 1−n p V − p V R(T − T ) w = 2 2 2 1 1 1 = 2 2 1 1 = 2 1 n − 1 n − 1 n − 1 where the last equality follows from the ideal gas law pV = RT and our deriva- tion for the work of a polytropic process is complete. -

MECH 230 – Thermodynamics 1 Final Workbook Solutions
MECH 230 – Thermodynamics 1 Final Workbook Solutions CREATED BY JUSTIN BONAL MECH 230 Final Exam Workbook Contents 1.0 General Knowledge ........................................................................................................................... 1 1.1 Unit Analysis .................................................................................................................................. 1 1.2 Pressure ......................................................................................................................................... 1 2.0 Energies ............................................................................................................................................ 2 2.1 Work .............................................................................................................................................. 2 2.2 Internal Energy .............................................................................................................................. 3 2.3 Heat ............................................................................................................................................... 3 2.4 First Law of Thermodynamics ....................................................................................................... 3 3.0 Ideal gas ............................................................................................................................................ 4 3.1 Universal Gas Constant ................................................................................................................. -

A Turbo-Brayton Cryocooler for Future European Observation Satellite Generation
C19_078 1 A Turbo-Brayton Cryocooler for Future European Observation Satellite Generation J. Tanchon1, J. Lacapere1, A. Molyneaux2, M. Harris2, S. Hill2, S.M. Abu-Sharkh2, T. Tirolien3 1Absolut System SAS, Seyssinet-Pariset, France 2Ofttech, Gloucester, United Kingdom 3European Space Agency, Noordwijk, The Netherlands ABSTRACT Several types of active cryocoolers have been developed for space and military applications in the last ten years. Performances and reliability continuously increase to follow the requirements evolution of new generations of satellite: less power consumption, more cooling capacity, increased life duration. However, in addition to this increase of performances and reliability, the microvibrations re- quirement becomes critical. In fact, with the development of vibration-free technologies, classical Earth Observation cryocoolers (Stirling, Pulse Tube) will become the main source of microvibra- tions on-board the satellite. A new generation cryocooler is being developed at Absolut System using very high speed turbomachines in order to avoid any generated perturbations below 1000 Hz. This development is performed in the frame of ESA Technical Research Program - 4000113495/15/NL/KML. This paper presents the status of this development project based on Reverse Brayton cycle with very high speed turbomachines. INTRODUCTION The space cryogenics sector is characterized by a large number of applications (detection, im- aging, sample conservation, propulsion, telecommunications etc.) that lead to various requirements (temperature range, microvibration, lifetime, power consumption etc.) that can be met by different solutions (Stirling or JT coolers, mechanical or sorption compressors etc.). The recent years saw, in Europe, the developments of coolers to meet Earth Observation mission UHTXLUHPHQWVWKDWDUHFDSDEOHWRSURYLGHVLJQL¿FDQWFRROLQJSRZHUDWDQRSHUDWLRQDOWHPSHUDWXUH around 50K (for IR detection). -

3. Thermodynamics Thermodynamics
Design for Manufacture 3. Thermodynamics Thermodynamics • Thermodynamics – study of heat related matter in motion. • Ma jor deve lopments d uri ng 1650 -1850 • Engineering thermodynamics mainly concerned with work produc ing or utili si ng machi nes such as - Engines, - TbiTurbines and - Compressors together w ith th e worki ng sub stances used i n th e machi nes. • Working substance – fluids: capable of deformation, energy transfer • Air an d s team are common wor king sub st ance Design and Manufacture Pressure F P = A Force per unit area. Unit: Pascal (Pa) N / m 2 1 Bar =105 Pa 1 standard atmosphere = 1.01325 Bar 1 Bar =14.504 Psi (pound force / square inch, 1N= 0.2248 pound force) 1 Psi= 6894.76 Pa Design and Manufacture Phase Nature of substance. Matter can exists in three phases: solid, liquid and gas Cycle If a substance undergoes a series of processes and return to its original state, then it is said to have been taken through a cycle. Design for Manufacture Process A substance is undergone a process if the state is changed by operation carried out on it Isothermal process - Constant temperature process Isobaric process - Constant pressure process Isometric process or isochoric process - Constant volume process Adiabatic Process No heat is transferred, if a process happens so quickly that there is no time to transfer heat, or the system is very well insulated from its surroundings. Polytropic process Occurs with an interchange of both heat and work between the system and its surroundings E.g. Nonadiabatic expansion or compression -

First and Second Law Evaluation of Combined Brayton-Organic Rankine Power Cycle
Journal of Thermal Engineering, Vol. 6, No. 4, pp. 577-591, July, 2020 Yildiz Technical University Press, Istanbul, Turkey FIRST AND SECOND LAW EVALUATION OF COMBINED BRAYTON-ORGANIC RANKINE POWER CYCLE Önder Kaşka1, Onur Bor2, Nehir Tokgöz3* Muhammed Murat Aksoy4 ABSTRACT In the present work, we have conducted thermodynamic analysis of an organic Rankine cycle (ORC) using waste heat from intercooler and regenerator in Brayton cycle with intercooling, reheating, and regeneration (BCIRR). First of all, the first law analysis is used in this combined cycle. Several outputs are revealed in this study such as the cycle efficiencies in Brayton cycle which is dependent on turbine inlet temperature, intercooler pressure ratios, and pinch point temperature difference. For all cycles, produced net power is increased because of increasing turbine inlet temperature. Since heat input to the cycles takes place at high temperatures, the produced net power is increased because of increasing turbine inlet temperature for all cycles. The thermal efficiency of combined cycle is higher about 11.7% than thermal efficiency of Brayton cycle alone. Moreover, the net power produced by ORC has contributed nearly 28650 kW. The percentage losses of exergy for pump, turbine, condenser, preheater I, preheater II, and evaporator are 0.33%, 33%, 22%, 23%, 6%, and16% respectively. The differences of pinch point temperature on ORC net power and efficiencies of ORC are investigated. In addition, exergy efficiencies of components with respect to intercooling pressure ratio and evaporator effectiveness is presented. Exergy destructions are calculated for all the components in ORC. Keywords: Brayton Cycle, Organic Rankine Cycle, Bottoming Cycle, Pinch Point Temperature, Waste Heat, Energy and Exergy Analysis INTRODUCTION Thermal energy systems as well as corresponding all parts have been challenged to improve overall efficiency due to lack of conventional fuels, reduce climate change and so on for recent years. -

Chapter 7 BOUNDARY WORK
Chapter 7 BOUNDARY WORK Frank is struck - as if for the first time - by how much civilization depends on not seeing certain things and pretending others never occurred. − Francine Prose (Amateur Voodoo) In this chapter we will learn to evaluate Win, which is the work term in the first law of thermodynamics. Work can be of different types, such as boundary work, stirring work, electrical work, magnetic work, work of changing the surface area, and work to overcome friction. In this chapter, we will concentrate on the evaluation of boundary work. 110 Chapter 7 7.1 Boundary Work in Real Life Boundary work is done when the boundary of the system moves, causing either compression or expansion of the system. A real-life application of boundary work, for example, is found in the diesel engine which consists of pistons and cylinders as the prime component of the engine. In a diesel engine, air is fed to the cylinder and is compressed by the upward movement of the piston. The boundary work done by the piston in compressing the air is responsible for the increase in air temperature. Onto the hot air, diesel fuel is sprayed, which leads to spontaneous self ignition of the fuel-air mixture. The chemical energy released during this combustion process would heat up the gases produced during combustion. As the gases expand due to heating, they would push the piston downwards with great force. A major portion of the boundary work done by the gases on the piston gets converted into the energy required to rotate the shaft of the engine. -

Design of a Cryogenic Turbine for a Hybrid Cryocooler
Design of a Cryogenic Turbine for a Hybrid Cryocooler by Thomas L. Fraser A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science (Mechanical Engineering) at the UNIVERISTY OF WISCONSIN-MADISON 2006 Approved by _____________________________________________ ______________ Professor Gregory F. Nellis Date i Abstract The hybrid pulse tube-reverse Brayton cycle cryocooler has the potential for cooling to temperatures on the order of 10 K. By using the rectifying interface which converts the oscillating pulse tube flow to continuous flow, both vibrations and low temperature regenerator losses are overcome, making the hybrid an ideal candidate for cooling infrared focal plane arrays which demand low temperature and low vibration. However, the turboexpander within the reverse Brayton cycle is complex and its performance is highly dependent on the performance of its subcomponents, thus necessitating a model predicting the turboexpander performance. A model was developed to predict the performance of the reverse Brayton cycle stage including the recuperative heat exchanger and turboexpander components. The turboexpander was numerically modeled in detail to include the sub-models of rotordynamics, the thermal and leakage performance of the seal, and the turboalternator. Where possible, the models were verified against either an analytical model or experimental data. A parametric analysis was carried out to determine the optimal design and conditions for the turboexpander. ii Acknowledgements First and foremost I would like to thank Greg Nellis. Besides being my advisor for this project he is also responsible for sparking an interest in energy science through his heat transfer courses I took as an undergrad. -

Heat Transfer During the Piston-Cylinder Expansion of a Gas
AN ABSTRACT OF THE THESIS OF Matthew Jose Rodrigues for the degree of Master of Science in Mechanical Engineering presented on June 5, 2014. Title: Heat Transfer During the Piston-Cylinder Expansion of a Gas Abstract approved: ______________________________________________________ James A. Liburdy The expansion of a gas within a piston-cylinder arrangement is studied in order to obtain a better understanding of the heat transfer which occurs during this process. While the situation of heat transfer during the expansion of a gas has received considerable attention, the process is still not very well understood. In particular, the time dependence of heat transfer has not been well studied, with many researchers instead focused on average heat transfer rates. Additionally, many of the proposed models are not in agreement with each other and are usually dependent on experimentally determined coefficients which have been found to vary widely between test geometries and operating conditions. Therefore, the expansion process is analyzed in order to determine a model for the time dependence of heat transfer during the expansion. A model is developed for the transient heat transfer by assuming that the expansion behaves in a polytropic manner. This leads to a heat transfer model written in terms of an unknown polytropic exponent, n. By examining the physical significance of this parameter, it is proposed that the polytropic exponent can be related to a ratio of the time scales associated with the expansion process, such as a characteristic Peclet number. Experiments are conducted to test the proposed heat transfer model and to establish a relationship for the polytropic exponent. -

Supercritical Carbon Dioxide(S-CO2) Power Cycle for Waste Heat Recovery: a Review from Thermodynamic Perspective
processes Review Supercritical Carbon Dioxide(s-CO2) Power Cycle for Waste Heat Recovery: A Review from Thermodynamic Perspective Liuchen Liu, Qiguo Yang and Guomin Cui * School of Energy and Power Engineering, University of Shanghai for Science and Technology, Shanghai 200093, China; [email protected] (L.L.); [email protected] (Q.Y.) * Correspondence: [email protected] Received: 11 October 2020; Accepted: 13 November 2020; Published: 15 November 2020 Abstract: Supercritical CO2 power cycles have been deeply investigated in recent years. However, their potential in waste heat recovery is still largely unexplored. This paper presents a critical review of engineering background, technical challenges, and current advances of the s-CO2 cycle for waste heat recovery. Firstly, common barriers for the further promotion of waste heat recovery technology are discussed. Afterwards, the technical advantages of the s-CO2 cycle in solving the abovementioned problems are outlined by comparing several state-of-the-art thermodynamic cycles. On this basis, current research results in this field are reviewed for three main applications, namely the fuel cell, internal combustion engine, and gas turbine. For low temperature applications, the transcritical CO2 cycles can compete with other existing technologies, while supercritical CO2 cycles are more attractive for medium- and high temperature sources to replace steam Rankine cycles. Moreover, simple and regenerative configurations are more suitable for transcritical cycles, whereas various complex configurations have advantages for medium- and high temperature heat sources to form cogeneration system. Finally, from the viewpoints of in-depth research and engineering applications, several future development directions are put forward. This review hopes to promote the development of s-CO2 cycles for waste heat recovery. -
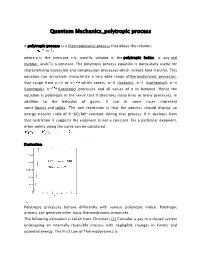
Quantum Mechanics Polytropic Process
Quantum Mechanics_polytropic process A polytropic process is a thermodynamic process that obeys the relation: where p is the pressure, v is specific volume, n, the polytropic index, is any real number, andC is a constant. The polytropic process equation is particularly useful for characterizing expansion and compression processes which include heat transfer. This equation can accurately characterize a very wide range ofthermodynamic processes, that range from n<0 to n= which covers, n=0 (Isobaric), n=1 (Isothermal), n=γ (Isentropic), n= (Isochoric) processes and all values of n in between. Hence the equation is polytropic in the sense that it describes many lines or many processes. In addition to the behavior of gases, it can in some cases represent some liquids and solids. The one restriction is that the process should display an energy transfer ratio of K=δQ/δW=constant during that process. If it deviates from that restriction it suggests the exponent is not a constant. For a particular exponent, other points along the curve can be calculated: Derivation Polytropic processes behave differently with various polytropic indice. Polytropic process can generate other basic thermodynamic processes. The following derivation is taken from Christians.[1] Consider a gas in a closed system undergoing an internally reversible process with negligible changes in kinetic and potential energy. The First Law of Thermodynamics is Define the energy transfer ratio, . For an internally reversible process the only type of work interaction is moving boundary work, given by Pdv. Also assume the gas is calorically perfect (constant specific heat) so du = cvdT. The First Law can then be written (Eq. -

Gas Power Cycles
Week 11 Gas Power Cycles ME 300 Thermodynamics II 1 Today’s Outline • Gas turbine engines • Brayton cycle • Analysis • Example ME 300 Thermodynamics II 2 Gas Turbine Engine • Produces shaft power by GE H series power generation gas turbine. This 480-megawatt unit has a rated thermal expanding high enthalpy gas efficiency of 60% in combined cycle through a turbine configurations. • A compressor and a combustor produce the enthalpy increasing pressure and temperature, resp. • The spinning turbine rotates a shaft which drives the compressor • Remaining enthalpy can be used to drive a generator or can be expanded in a nozzle producing kinetic energy e.g. thrust ME 300 Thermodynamics II 3 Enthalpy Generation and Conversion Turbine Fuel converts enthalpy Generator to shaft power for Compressor Combustor electricity increases increases pressure Temperature Air (pv) (u) Nozzle converts enthalpy into kinetic energy ME 300 Thermodynamics II 4 Gas Turbine Engine Components http://www.stanford.edu/group/ctr/ResBriefs/temp05/schluter2.pdf www.aem.umn.edu/research/Images/pw_GasTurbine.gif ME 300 Thermodynamics II 5 Combustor Simulations ME 300 Thermodynamics II 6 Schematic • Fresh air enters compressors ~constant pressure • Air compressed to high pressure in compressor e.g. 23:1 • High pressure air is mixed with injected fuel spray in combustor raising temperatures • High enthalpy product gases expand in turbine producing shaft work to run compressor or Open cycle generator ME 300 Thermodynamics II 7 Air Standard Brayton Cycle • Model as closed cycle