Hybridization and Sexual Reproduction in the Invasive Alien Fallopia (Polygonaceae) Complex in Belgium
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Perceptions of Risk from Non-Native and Horticultural Plants
Research Collection Doctoral Thesis Perceptions of Risk from Non-Native and Horticultural Plants Author(s): Humair Kuhn, Franziska Publication Date: 2014 Permanent Link: https://doi.org/10.3929/ethz-a-010252721 Rights / License: In Copyright - Non-Commercial Use Permitted This page was generated automatically upon download from the ETH Zurich Research Collection. For more information please consult the Terms of use. ETH Library DISS. ETH NO. 22073 Perceptions of Risk from Non-Native and Horticultural Plants A thesis submitted to attain the degree of DOCTOR OF SCIENCES of ETH ZURICH (Dr. sc. ETH Zurich) presented by FRANZISKA HUMAIR KUHN M.Sc. in Biology, University of Basel, Switzerland born on November 10, 1968 citizen of Basel (BS), Escholzmatt-Marbach (LU), Waltenschwil (AG) accepted on the recommendation of Prof. Dr. Michael Siegrist, examiner Prof. Dr. Peter Edwards, co-examiner Prof. Dr. Petra Lindemann-Matthies, co-examiner PD Dr. Christoph Kueffer Schumacher, co-examiner 2014 3 5 Summary 1. The life of humans is inextricably linked to biodiversity and functioning ecosys- tems. Nevertheless, we are in the process of changing our planet to such an extent that many species and species communities are critically endangered. The intro- duction of new, non-native plant species to established ecosystems is perceived as one of the main threats to global biodiversity: Some of these species may become dominant and lead to novel interactions within ecosystems (plant invasions). Hu- mans are the main driver of plant invasions. In order to better understand the invasion process, not only ecological relationships, but also human motivation be- hind the choice to introduce certain species has to be examined. -

Mendelova Univerzita V Brně Zahradnická Fakulta V Lednici
Mendelova univerzita v Brně Zahradnická fakulta v Lednici Použití pnoucích rostlin na území České republiky v první polovině 20. století Diplomová práce Vedoucí diplomové práce: Vypracovala: prof. Ing. Miloš Pejchal, CSc. Bc. Andrea Dundáčková Lednice 2017 2 Čestné prohlášení Prohlašuji, že jsem diplomovou práci na téma Použití pnoucích rostlin na území České republiky v první polovině 20. století vypracovala samostatně a veškeré použité prameny a informace uvádím v seznamu použité literatury. Souhlasím, aby moje práce byla zveřejněna v souladu s § 47b zákona č. 111/1998 Sb., o vysokých školách a o změně a doplnění dalších zákonů (zákon o vysokých školách), ve znění pozdějších předpisů, a v souladu s platnou Směrnicí o zveřejňování vysokoškolských závěrečných prací. Jsem si vědoma, že se na moji práci vztahuje zákon č. 121/2000 Sb., autorský zákon, a že Mendelova univerzita v Brně má právo na uzavření licenční smlouvy a užití této práce jako školního díla podle § 60 odst. 1 autorského zákona. Dále se zavazuji, že před sepsáním licenční smlouvy o využití díla jinou osobou (subjektem) si vyžádám písemné stanovisko univerzity, že předmětná licenční smlouva není v rozporu s oprávněnými zájmy univerzity, a zavazuji se uhradit případný příspěvek na úhradu nákladů spojených se vznikem díla, a to až do jejich skutečné výše. V Lednici, dne 10. 5. 2017 Podpis studenta ………………………………. Bc. Andrea Dundáčková 3 Poděkování Děkuji především panu prof. Ing. Miloši Pejchalovi, CSc. za odborné vedení, trpělivost, ochotu a za čas strávený konzultacemi nad tématem diplomové práce. Dále bych chtěla velice poděkovat Ústavu biotechniky zeleně Zahradnické fakulty Mendelovy univerzity v Brně za ochotné poskytnutí badatelských zdrojů. -

Forest Health Technology Enterprise Team Biological Control of Invasive
Forest Health Technology Enterprise Team TECHNOLOGY TRANSFER Biological Control Biological Control of Invasive Plants in the Eastern United States Roy Van Driesche Bernd Blossey Mark Hoddle Suzanne Lyon Richard Reardon Forest Health Technology Enterprise Team—Morgantown, West Virginia United States Forest FHTET-2002-04 Department of Service August 2002 Agriculture BIOLOGICAL CONTROL OF INVASIVE PLANTS IN THE EASTERN UNITED STATES BIOLOGICAL CONTROL OF INVASIVE PLANTS IN THE EASTERN UNITED STATES Technical Coordinators Roy Van Driesche and Suzanne Lyon Department of Entomology, University of Massachusets, Amherst, MA Bernd Blossey Department of Natural Resources, Cornell University, Ithaca, NY Mark Hoddle Department of Entomology, University of California, Riverside, CA Richard Reardon Forest Health Technology Enterprise Team, USDA, Forest Service, Morgantown, WV USDA Forest Service Publication FHTET-2002-04 ACKNOWLEDGMENTS We thank the authors of the individual chap- We would also like to thank the U.S. Depart- ters for their expertise in reviewing and summariz- ment of Agriculture–Forest Service, Forest Health ing the literature and providing current information Technology Enterprise Team, Morgantown, West on biological control of the major invasive plants in Virginia, for providing funding for the preparation the Eastern United States. and printing of this publication. G. Keith Douce, David Moorhead, and Charles Additional copies of this publication can be or- Bargeron of the Bugwood Network, University of dered from the Bulletin Distribution Center, Uni- Georgia (Tifton, Ga.), managed and digitized the pho- versity of Massachusetts, Amherst, MA 01003, (413) tographs and illustrations used in this publication and 545-2717; or Mark Hoddle, Department of Entomol- produced the CD-ROM accompanying this book. -

Replace This with the Actual Title Using All Caps
SYSTEMATICS OF ANTIGONON AND TROPICAL ERIOGONOIDEAE: PHYLOGENY, TAXONOMY, AND INVASION BIOLOGY A Dissertation Presented to the Faculty of the Graduate School of Cornell University In Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Janelle Marie Burke May 2011 © 2011 Janelle Marie Burke SYSTEMATICS OF ANTIGONON AND TROPICAL ERIOGONOIDEAE: PHYLOGENY, TAXONOMY, AND INVASION BIOLOGY Janelle Marie Burke, Ph. D. Cornell University 2011 The genera of Polygonaceae have historically been segregated into two subfamilies, Eriogonoideae and Polygonoideae, based on a few key morphological characters. Using ITS, morphology and five chloroplast markers, a phylogeny for Eriogonoideae was reconstructed, with an emphasis on sampling of the tropical genera. Results support the placement of nine of twelve woody, tropical genera within Eriogonoideae, where these genera form a paraphyletic assemblage giving rise to Eriogoneae (Eriogonum and allies). My work corroborates previous phylogenetic studies, and suggests a broader circumscription of Eriogonoideae. Also based on these results, I propose the resurrection of a third subfamily, Symmerioideae, in Polygonaceae, and propose two new tribes, Gymnopodieae and Leptogoneae, in Eriogonoideae. Within the subfamily, the genus Antigonon provides a systematic challenge. Although Antigonon is a small, easily-recognized genus, the boundaries of species within it have never been resolved satisfactorily. A taxonomic treatment for the genus is presented, based on morphology and molecular phylogenetic data from two chloroplast markers (psaI-accD, psbA-trnH ) and one nuclear marker (LFY , 2nd intron). Four species are described, and a new subspecies, Antigonon leptopus subsp. coccineum is proposed. Antigonon leptopus is also known as corallita, a pantropical invasive vine particularly problematic on islands. -

Contributions to the Pharmacognostical and Phytobiological Study of Fallopia Aubertii (L. Henry) Holub. (Polygonaceae)
FARMACIA, 2013, Vol. 61, 5 991 CONTRIBUTIONS TO THE PHARMACOGNOSTICAL AND PHYTOBIOLOGICAL STUDY OF FALLOPIA AUBERTII (L. HENRY) HOLUB. (POLYGONACEAE) OCTAVIAN TUDOREL OLARU1, ADRIANA IULIANA ANGHEL*1, VIORICA ISTUDOR2, ROBERT VIOREL ANCUCEANU1, MIHAELA DINU1 1Pharmaceutical Botany Department, “Carol Davila” University of Medicine and Pharmacy, 6 Traian Vuia, 020956, Bucharest, Romania 2Pharmacognosy. Phytochemistry. Phytotherapy Department, “Carol Davila” University of Medicine and Pharmacy, 6 Traian Vuia, 020956, Bucharest, Romania *corresponding author: [email protected] Abstract In order to evaluate the therapeutic potential of the Faloppia aubertii (L.Henry) Holub (Russian vine) species for the use of the flowered aerial parts as raw material in the preparation of an active pharmacological extract, we decided to establish the microscopic characters, to identify the chemical constituents and to verify the cytotoxicity of this herbal product. The root and the stem are characterized by secondary structure with a well developed secondary xylem and sclerenchyma; the leaf blade - dorsi-ventral structure with two vascular bundles and calcium oxalate druses at the main vein, pluricellular glandular trichoma and anomocytic stomata within the epidermis; druses of calcium oxalate are present in all the organs examined. The following classes of phytochemicals were identified: carotenoids, flavonoids, sterols/triterpenes, polyphenol carboxylic acids, condensed tannins, polysaccharides, proanthocyanidins and reductive compounds. Through -

108 Anastasiu & Negrean
590 Plant, fungal and habitat diversity investigation and conservation • Proceedings of IV BBC – Sofia ' 2006 Alien vascular plants in Dobrogea (Romania) and their impact on different types of habitats Paulina Anastasiu & Gavril Negrean University of Bucharest, Faculty of Biology, 1–3, Aleea Portocalelor St., 060110– Bucureşti–35, Romania, e-mail: [email protected] (corresponding author) Abstract. In different types of habitats from Dobrogea (including Danube Delta) we identified 140 neophytes and 9 archaeophytes; about half of them are from America and arrived here accidentally. Among neophytes, 30 taxa have invasive status, but only few are aggressive having a negative impact on different ecosystems: Ailanthus altissima, Amorpha fruticosa, Azolla filiculoides, Bidens frondosa, Conyza canadensis, Elodea nuttallii, Lindernia dubia, Paspalum paspalodes. Key words: Dobrogea, ecosystems, impact, invasive alien plants, Romania Introduction (Brândză 1898), Elodea canadensis (Macovei & Scrib- an 1905), Lycium barbarum (Grecescu 1909) and Azolla Dobrogea (including Danube Delta) is a very important filiculoides (Pallis 1916). The last records are: Bellardia region for plant (about 2000 taxa) and habitat diversi- trixago (Ciocârlan & Costea 2004), Chloris barbata and ty (wetlands, dry grasslands, calcareous stony slopes, Impatiens balsamina (Anastasiu & Negrean 2005). thermophilous woodlands, dunes and sands habitats, etc.). Here there are 20 endemic taxa and over 200 rare Material and methods plants, many of them included in IUCN Red list, Bern Convention, Habitats Directive (Campanula romanica, A comprehensive database with neophytes recorded Centaurea jankae, C. pontica, Salicornia veneta, Achil- from Dobrogea (including Danube Delta) has been lea thracica, Aldrovanda vesiculosa, Alyssum borzaea- done. The data were obtained from literature, herbar- num, Colchicum fominii, Liparis loeselii, Marsilea quad- ium sheets and as a result of own field observations rifolia, Moehringia jankae, Paeonia tenuifolia, Potentilla along many years. -

Invasive Species in Botanical Garden of Ajuda (Portugal)
EuroGard IV - Botanic Gardens and the 2010 Challenge 18-22 September 2006 PRUHONICE, THE CZECH REPUBLIC Invasive species in Botanical Garden of Ajuda (Portugal) Dalila Espírito-Santo, Ana Monteiro, Teresa Vasconcelos & Ilídio Moreira Instituto Superior de Agronomia, Tapada da Ajuda, 1349-017 Lisboa, Portugal, [email protected], [email protected], [email protected]; [email protected] Introduction Botanical gardens have been identified as a source of invasive plants in the past and have often contributed to its spread in part because they answer a great number of requests they get from around the world to distribute their plants or seeds. Botanists, conservationists, foresters, agroforesters and horticulturalists have, and often still are, to varying degrees, responsible for the introduction and planting of woody alien species, but there is no evidence to show that scientists responsible for their introduction were aware of the potential problems. Many invasive plants have been introduced by their uses as food or as medicine but also as ornamentals and they continue to be admired by gardeners who may not be aware of their weedy nature. Many of the qualities that make plants valuable in gardens also make them potentially invasive. Easy germination and propagation, hardiness, rapid growth, abundant flowers, and resistance to insects and diseases are qualities that are desirable in ornamental plants, yet also are characteristics of weedy plants. The Botanical Garden richness is due to the introduction of species from around the world but it is known that some species became invasive under new environmental conditions. This is very clear at the Botanical Garden of Ajuda where some species do not become invasive until they are neglected for a long time. -

Din Arad Facultatea De Ştiinţe Ale Naturii Flora
UNIVERSITATEA DE VEST ”VASILE GOLDIŞ” DIN ARAD FACULTATEA DE ŞTIINŢE ALE NATURII IOAN DON FLORA LEMNOASĂ SPONTANĂ ŞI CULTIVATĂ DIN ZONA ARADULUI Rezumatul tezei de doctorat Conducător ştiinţific Prof. Univ. Dr. AUREL ARDELEAN -Arad, 2011- CUPRINS INTRODUCERE ............................................................................................................. 3 SCOPUL CERCETĂRILOR ........................................................................................... 3 OBIECTIVELE URMĂRITE .......................................................................................... 3 ZONA LUATĂ ÎN STUDIU............................................................................................ 4 ISTORICUL CERCETĂRILOR DENDO-BOTANICE.................................................. 4 STADIUL ACTUAL AL CUNOŞTINŢELOR ............................................................... 4 CADRUL FIZICO-GEOGRAFIC AL TERITORIULUI STUDIAT .............................. 4 MATERIAL ŞI METODĂ .............................................................................................. 4 REZULTATE ŞI DISCUŢII ............................................................................................ 5 CONCLUZII .................................................................................................................... 20 BIBLIOGRAFIE SELECTIVĂ ....................................................................................... 21 ANEXA – INDEXUL ALFABETIC AL GENURILOR ................................................ 22 2 INTRODUCERE -

ISSN 1387-3547, Volume 12, Number 10
ISSN 1387-3547, Volume 12, Number 10 This article was published in the above mentioned Springer issue. The material, including all portions thereof, is protected by copyright; all rights are held exclusively by Springer Science + Business Media. The material is for personal use only; commercial use is not permitted. Unauthorized reproduction, transfer and/or use may be a violation of criminal as well as civil law. Biol Invasions (2010) 12:3525–3549 Author's personal copy DOI 10.1007/s10530-010-9749-0 ORIGINAL PAPER The alien flora of Greece: taxonomy, life traits and habitat preferences Margarita Arianoutsou • Ioannis Bazos • Pinelopi Delipetrou • Yannis Kokkoris Received: 13 May 2009 / Accepted: 29 March 2010 / Published online: 18 July 2010 Ó Springer Science+Business Media B.V. 2010 Abstract The aim of the paper is the state-of-the-art the native flora. Regarding flowering traits, most of assessment of the alien flora of Greece and its traits. the aliens have a long flowering period (over The dataset consists of a total of 343 alien taxa, 1 month) and flower in late spring, summer and including 49 archaeophytes. The taxonomy, life traits autumn, when few of the native plants are in bloom. and habitat of the 294 neophytes are analysed vs their Vertebrate zoochory and anemochory are the two naturalisation status. Out of the 122 (41%) natura- dispersal modes mostly utilised by the alien plants lised neophytes, 50 are identified as exhibiting (43 and 28%, respectively), while more than one invasive behaviour. Poaceae, Asteraceae, Amaranth- dispersal mechanisms are functional for 56% of them. -

Rod Fallopia Adans. V Sloveniji
Hladnikia 28: 17-40 (2011) 17 Rod Fallopia Adans. v Sloveniji The genus Fallopia Adans. in Slovenia SIMONA STRGULC KRAJšEK & NEJC JOGAN Oddelek za biologijo, Biotehniška fakulteta, Univerza v Ljubljani, Večna pot 111, 1000 Ljubljana, Slovenija. [email protected]; [email protected] Izvleček Članek obravnava slakovce in dresnike (skupaj v rodu Fallopia) v Sloveniji, njihovo razlikovanje, pojavljanje, medsebojna križanja vrst, razširjenost in ekološke zahteve. Poseben poudarek je na dresnikih, pri katerih je poleg že znanih vrst japonskega (F. japonica) in sahalinskega (F. sachalinensis) prvič podrobneje predstavljen češki dresnik (F. x bohemica), križanec med prej omenjenima, ki pa oblikuje tudi samostojne populacije. Vse tri vrste dresnika so tujerodne invazivne vrste, zato je predstavljena tudi problematika njihovega širjenja in možnosti odstranjevanja. Abstract Article deals with bindweeds and knotweeds (together in genus Fallopia) in Slovenia, their distinguishing characters, occurrence, hybridization, distribution and ecology. Special attention is paid to knotweeds and in addition to already known Japanese (F. japonica) and giant knotweed (F. sachalinensis), their hybrid, F. x bohemica, which was recorded in Slovenia just recently and forms monospecific stands without parental species, is discussed for the first time in Slovenia. All of the three knotweed taxa are invasive aliens, so their invasiveness, spreading and potential methods for control are also discussed. 1 Uvod Rod Fallopia Adans. (incl. Reynoutria Houtt.) je eden izmed 46 rodov družine dresnovk (Polygonaceae) (MABBERLEY 1997), ki ga slovensko imenujemo slakovec (ovijalke) ali dresnik (grmasto razrasli predstavniki). Rod je v zadnjem času pri nas najbolj znan zaradi izredno agresivnega invazivnega japonskega dresnika (Fallopia japonica (Houtt.) Ronse Decraene), ki velja za eno izmed 100 najhujših invazivk v svetovnem merilu (LOWE & al. -
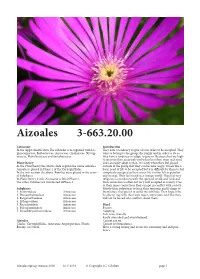
Aizoales 3-663.20.00
Aizoales 3-663.20.00 Taxonomy Introduction In the Apg2 classifcation Te suborder is recognised with Lo- Tey have a tendency to give a lot in order to be accepted. Tey phiocarpaceae, Barbeuiaceae, Aizoaceae, Gisekiaceae, Nyctag- want to belong to the group, the family and in order to do so inaceae, Phytolaccaceae and Sarcobataceae. they have a tendency to adapt, to give in. Because they are high- ly sensitive they accurately feel what the others want and need Plant theory and can easily adapt to that. It is only when they feel placed In the Plant theory the above clade is given the name Aizoales. outside of the group that they can become angry. It feels like a Aizoales is placed in Phase 2 of the Caryophyllidae. basic need of life to be accepted but it is difcult for them to feel In the frst version the above Families were placed in the sever- completely accepted as their inner life is ofen felt as peculiar al Subphases. and strange. Tey feel weird in a strange world. Tey feel very In Plant theory 2 only Aizoaceae is lef inPhase 2. religious, a connection with the spiritual world and God and Te other Families are transferred toPhase 3. that connection is ofen not very well accepted in society. Due to their inner convictions they can get in confict with society. Subphases Mostly their solution is to keep their opinions and feelings to 1. Sesuvioideae Aizoaceae themselves; they prefer to avoid the conficts. Tey hope to be 2. Drosanthemoideae Aizoaceae be able to stay with their own inner convictions and that they 3. -
Synonymique De La Flore De Suisse Synonymie-Index Der Schweizer Flora Indice Sinonimico Della Flora Della Svizzera
Documenta Floristicae Helvetiae N° 2 Index synonymique de la Flore de Suisse et territoires limitrophes (ISFS) Synonymie-Index der Schweizer Flora und der angrenzenden Gebiete (SISF) Indice sinonimico della Flora della Svizzera e territori limitrofi (ISFS) D. Aeschimann & C. Heitz avec la collaboration de – in Zusammenarbeit mit – con la collaborazione di C. Latour, P. Perret & B. Bäumler 2ème édition – 2te Auflage – 2da edizione Genève 2005 Abréviations Statut des noms latins Ouvrages de référence aggr. = [lat.: aggregatum], agrégat 1. Les noms Acceptés, retenus par les auteurs de l’ISFS. Ils Referenzwerke auct. = [lat.: auctorum], des auteurs figurent en gras (ex.: Sparganium angustifolium Michx.). Opere di riferimento s.l. = [lat.: sensu lato], au sens large 2. Les noms Synonymes, qui sont en italiques (ex.: Sparganium AB = Aeschimann, D. & H. M. Burdet (2005). Flore de la s.str. = [lat.: sensu stricto], au sens strict minimum Wallr.). Suisse et des territoires limitrophes. Le Nouveau Binz. subsp. = [lat.: subspecies], sous-espèce 3. Les noms appliqués à des taxons Inclus, qui correspondent Ed. 3. Haupt, Berne. var. = [lat.: varietas], variété à des entités taxonomiques étroites, incluses dans un taxon Ξ = xénophyte plus large au nom le plus souvent accepté. Ils sont cités en BH = Binz, A. & Ch. Heitz (1990). Schul- und Exkursions- X = pas présent en Suisse gras et italiques (ex.: Sparganium erectum subsp. oocarpum flora für die Schweiz. Ed. 19. Schwabe, Basel. ? = présence en Suisse douteuse, ou sporadique (Čelak.) Domin). FH = Lauber, K. & G. Wagner (2001). Flora Helvetica. 4. Les noms en Regroupant plusieurs autres. Ils figurent en Explications complètes aux pp. 5-6. Ed.