Insects and Other Arthropods As Agents of Vector-Dispersal in Fungi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Insecta: Coleoptera: Leiodidae: Cholevinae), with a Description of Sciaphyes Shestakovi Sp.N
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arthropod Systematics and Phylogeny Jahr/Year: 2011 Band/Volume: 69 Autor(en)/Author(s): Fresneda Javier, Grebennikov Vasily V., Ribera Ignacio Artikel/Article: The phylogenetic and geographic limits of Leptodirini (Insecta: Coleoptera: Leiodidae: Cholevinae), with a description of Sciaphyes shestakovi sp.n. from the Russian Far East 99-123 Arthropod Systematics & Phylogeny 99 69 (2) 99 –123 © Museum für Tierkunde Dresden, eISSN 1864-8312, 21.07.2011 The phylogenetic and geographic limits of Leptodirini (Insecta: Coleoptera: Leiodidae: Cholevinae), with a description of Sciaphyes shestakovi sp. n. from the Russian Far East JAVIER FRESNEDA 1, 2, VASILY V. GREBENNIKOV 3 & IGNACIO RIBERA 4, * 1 Ca de Massa, 25526 Llesp, Lleida, Spain 2 Museu de Ciències Naturals (Zoologia), Passeig Picasso s/n, 08003 Barcelona, Spain [[email protected]] 3 Ottawa Plant Laboratory, Canadian Food Inspection Agency, 960 Carling Avenue, Ottawa, Ontario, K1A 0C6, Canada [[email protected]] 4 Institut de Biologia Evolutiva (CSIC-UPF), Passeig Marítim de la Barceloneta, 37 – 49, 08003 Barcelona, Spain [[email protected]] * Corresponding author Received 26.iv.2011, accepted 27.v.2011. Published online at www.arthropod-systematics.de on 21.vii.2011. > Abstract The tribe Leptodirini of the beetle family Leiodidae is one of the most diverse radiations of cave animals, with a distribution centred north of the Mediterranean basin from the Iberian Peninsula to Iran. Six genera outside this core area, most notably Platycholeus Horn, 1880 in the western United States and others in East Asia, have been assumed to be related to Lepto- dirini. -

Agathidium Vaderi MILLER & WHEELER, 2005
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Linzer biologische Beiträge Jahr/Year: 2021 Band/Volume: 0052_2 Autor(en)/Author(s): Schifko Georg Artikel/Article: Agathidium vaderi MILLER & WHEELER, 2005 (Coleoptera, Leiodidae) – Ein wissenschaftlicher Artname im Spannungsfeld der Zoologie, der Ethnologie und der Populärkultur 1099-1104 Linzer biol. Beitr. 52/2 1099-1104 Februar 2021 Agathidium vaderi MILLER & WHEELER, 2005 (Coleoptera, Leiodidae) – Ein wissenschaftlicher Artname im Spannungsfeld der Zoologie, der Ethnologie und der Populärkultur Georg SCHIFKO A b s t r a c t : In recent times, names related to popular culture have increasingly been given to new discovered species. This article deals with the Agathidium vaderi beetle named in 2005 by Kelly B. Miller and Quentin D. Wheeler after the fictional character Darth Vader due to the postulated similarity of the beetle's head with Darth Vader's helmet which is ultimately based on Japanese Samurai helmet designs. Additionally, attention is drawn to the the mutual benefits available to the taxonomy as well as for the films in the Star Wars series due to the unconventual species name. Key Words: Agathidium vaderi, Star Wars, Japanese helmets, Taxonomy, Nomenclature Einleitung Im Japanischen wird der zur Familie der Blatthornkäfer (Scarabaeidae) gehörende Allomyrina dichotoma (LINNAEUS, 1771) als kabutomushi bezeichnet, was soviel wie "Helminsekt" (kabuto = Helm, mushi = Insekt) bedeutet und eine Anspielung auf japanische Samuraihelme darstellt. A. dichotoma bildet im Land der aufgehenden Sonne einen fest verankerten Bestandteil der Populärkultur und man lässt dort Männchen dieser Spezies gegeneinander kämpfen. In den Wettbewerben geht es darum, wessen Käfer die schwerste Last ziehen kann und um Spiele, bei denen man die Käfermännchen dazu bringt um ein Stück Wassermelone zu kämpfen (LAURENT 2001: 70) – somit "eine Insekten- version des Sumo-Ringens" (HERZOG 2012: 57). -

The Biodiversity of Flying Coleoptera Associated With
THE BIODIVERSITY OF FLYING COLEOPTERA ASSOCIATED WITH INTEGRATED PEST MANAGEMENT OF THE DOUGLAS-FIR BEETLE (Dendroctonus pseudotsugae Hopkins) IN INTERIOR DOUGLAS-FIR (Pseudotsuga menziesii Franco). By Susanna Lynn Carson B. Sc., The University of Victoria, 1994 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES (Department of Zoology) We accept this thesis as conforming To t(p^-feguired standard THE UNIVERSITY OF BRITISH COLUMBIA 2002 © Susanna Lynn Carson, 2002 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. 1 further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department The University of British Columbia Vancouver, Canada DE-6 (2/88) Abstract Increasing forest management resulting from bark beetle attack in British Columbia's forests has created a need to assess the impact of single species management on local insect biodiversity. In the Fort St James Forest District, in central British Columbia, Douglas-fir (Pseudotsuga menziesii Franco) (Fd) grows at the northern limit of its North American range. At the district level the species is rare (representing 1% of timber stands), and in the early 1990's growing populations of the Douglas-fir beetle (Dendroctonus pseudotsuage Hopkins) threatened the loss of all mature Douglas-fir habitat in the district. -
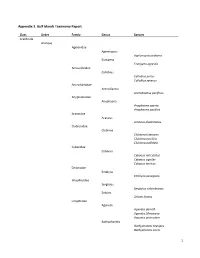
1 Appendix 3. Gulf Islands Taxonomy Report
Appendix 3. Gulf Islands Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Eratigena Eratigena agrestis Amaurobiidae Callobius Callobius pictus Callobius severus Antrodiaetidae Antrodiaetus Antrodiaetus pacificus Anyphaenidae Anyphaena Anyphaena aperta Anyphaena pacifica Araneidae Araneus Araneus diadematus Clubionidae Clubiona Clubiona lutescens Clubiona pacifica Clubiona pallidula Cybaeidae Cybaeus Cybaeus reticulatus Cybaeus signifer Cybaeus tetricus Dictynidae Emblyna Emblyna peragrata Gnaphosidae Sergiolus Sergiolus columbianus Zelotes Zelotes fratris Linyphiidae Agyneta Agyneta darrelli Agyneta fillmorana Agyneta protrudens Bathyphantes Bathyphantes brevipes Bathyphantes keeni 1 Centromerita Centromerita bicolor Ceratinops Ceratinops latus Entelecara Entelecara acuminata Erigone Erigone aletris Erigone arctica Erigone cristatopalpus Frederickus Frederickus coylei Grammonota Grammonota kincaidi Linyphantes Linyphantes nehalem Linyphantes nigrescens Linyphantes pacificus Linyphantes pualla Linyphantes victoria Mermessus Mermessus trilobatus Microlinyphia Microlinyphia dana Neriene Neriene digna Neriene litigiosa Oedothorax Oedothorax alascensis Pityohyphantes Pityohyphantes alticeps Pocadicnemis Pocadicnemis pumila Poeciloneta Poeciloneta fructuosa Saaristoa Saaristoa sammamish Scotinotylus Scotinotylus sp. 5GAB Semljicola Semljicola sp. 1GAB Sisicottus Spirembolus Spirembolus abnormis Spirembolus mundus Tachygyna Tachygyna ursina Tachygyna vancouverana Tapinocyba Tapinocyba -

(Lepidoptera: Papilionidae) of Kerala Part of Western Ghats Usin
Journal of Entomology and Zoology Studies 2014; 2 (4): 72-77 ISSN 2320-7078 Taxonomic segregation of the Swallowtails of the JEZS 2014; 2 (4): 72-77 © 2014 JEZS genus Graphium (Lepidoptera: Papilionidae) of Received: 23-06-2014 Accepted: 17-07-2014 Kerala part of Western Ghats using morphological V.S. Revathy characters of external genitalia Entomology Department, Forest Health Division, Kerala Forest V.S. Revathy and George Mathew Research Institute, Peechi, Kerala- 680635 Abstract George Mathew Studies on the genitalia of four species of Papilionids belonging to the tribe Leptocercini were made. The Entomology Department, Forest structure of vinculum, uncus, valvae and phallus of the male genitalia and the bursa, ductus and ovipositor Health Division, Kerala Forest of the female were found to be useful in taxonomic segregation of these butterflies. This highlights the Research Institute, Peechi, Kerala- extreme practical importance of external genitalic structures in the identification of these butterflies and 680635 improves upon earlier characters for generic and specific determinations based mainly on the wing venation, size and shape of palpi, and frons. Keywords: Taxonomy, Papilionidae, Lepidoptera, Graphium, Western Ghats 1. Introduction The Western Ghats constitute a mountain range along the western side of India. It is acclaimed as World Heritage Site by UNESCO and is one of the world’s eight “hottest hotspots" of biological diversity. Southern Western Ghats extending from the Agasthamalai to Palghat Gap has highest butterfly diversity with maximum Endemics. Thirty six species of butterflies are reported to be endemic to the Ghat and among the butterfly genera, the genus Parantirrhoea is exclusively [11] endemic to this region . -

Slime Molds: Biology and Diversity
Glime, J. M. 2019. Slime Molds: Biology and Diversity. Chapt. 3-1. In: Glime, J. M. Bryophyte Ecology. Volume 2. Bryological 3-1-1 Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 18 July 2020 and available at <https://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 3-1 SLIME MOLDS: BIOLOGY AND DIVERSITY TABLE OF CONTENTS What are Slime Molds? ....................................................................................................................................... 3-1-2 Identification Difficulties ...................................................................................................................................... 3-1- Reproduction and Colonization ........................................................................................................................... 3-1-5 General Life Cycle ....................................................................................................................................... 3-1-6 Seasonal Changes ......................................................................................................................................... 3-1-7 Environmental Stimuli ............................................................................................................................... 3-1-13 Light .................................................................................................................................................... 3-1-13 pH and Volatile Substances -
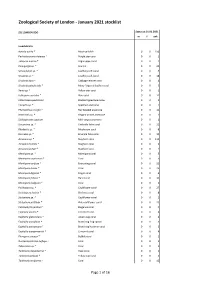
Jan 2021 ZSL Stocklist.Pdf (699.26
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp. -

How Nature Produces Blue Color
1 How Nature Produces Blue Color Priscilla Simonis and Serge Berthier Institut des Nanosciences de Paris (INSP), University Pierre et Marie Curie, Paris, France 1. Introduction Today, blue is a very fashionable color in European countries. This has not always been the case (Pastoureau, 2000), as cultural perceptions have slowly evolved since prehistoric times. In cave paintings, white, red and black have been the only available tones and these colors remained basic for Greek and Latin cultures, where blue was neglected or even strongly devalued. The word caeruleus, which is often used for brightly blue species, in naming plants and insects, is etymologically related to the word cera, which designates wax (not to the world caelum – sky – as often believed): it meant first white, brown or yellow (André, 1949), before being applied to green and black, and much lately, to a range of blues. Latin and Greek philosophers were so diverted from blue that they even did not notice its presence in the rainbow: for Anaximenes (585-528 BC) and later for Lucretius (98-55 BC), the rainbow only displayed red, yellow and violet; Aristotle (384-322 BC) and Epicurus (341-270 BC) described it as red, yellow, green and violet. Seneca (ca. 4 BC - 65 AD) only mentioned red, orange, green, violet but, strangely, also added purple, a metameric color not found in the decomposition of white light. Later in the Middle-Ages, Robert Grosseteste (ca. 1175-1253) revisited the rainbow phenomenon in its book “De Iride” and still did not find there any blue color (Boyer, 1954). Blue emerged slowly in minds and art, only after the advent of technological breakthroughs in stained glass fabrication (as introduced in the 12th century rebuild of St Denis Basilica) and after the progressive use of blue dyes, which followed the extension of woad cultivation, all after the 13th century. -

Speciation in Graphium Sarpedon (Linnaeus)
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Stuttgarter Beiträge Naturkunde Serie A [Biologie] Jahr/Year: 2013 Band/Volume: NS_6_A Autor(en)/Author(s): Page Malcolm G. P., Treadaway Colin G. Artikel/Article: Speciation in Graphium sarpedon (Linnaeus) and allies (Lepidoptera: Rhopalocera: Papilionidae) 223-246 Stuttgarter Beiträge zur Naturkunde A, Neue Serie 6: 223–246; Stuttgart, 30.IV.2013 223 Speciation in Graphium sarpedon (Linnaeus) and allies (Lepidoptera: Rhopalocera: Papilionidae) MALCOLM G. P. PAGE & COLIN G. TREADAWAY Abstract The relationships between subspecies of Graphium sarpedon (Linnaeus, 1758) and closely allied species have been investigated. This group appears to comprise eight sibling species, as follows: Graphium sarpedon (Linnaeus, 1758), G. adonarensis (Rothschild, 1896) stat. rev., G. anthedon (Felder & Felder, 1864), G. milon (Felder & Felder, 1865) stat. rev., G. isander (Godman & Salvin, 1888) stat. rev., G. choredon (Felder & Felder, 1864) stat. rev., G. teredon (Felder & Felder, 1864) stat. rev., G. jugans (Rothschild, 1896) stat. rev. The following new subspecies are described: G. sarpedon sirkari n. subsp. from N. India and S. China, G. adonarensis hundertmarki n. subsp. from Bali, G. adonarensis agusyantoei n. subsp. from Sumatra, G. adonarensis phyrisoides n. subsp. from Bangka and Belitung, G. adonarensis toxopei n. subsp. from Lombok, G. adonarensis septentrionicolus n. subsp. from N. India and E. China. K e y w o r d s : Lepidoptera, Papilionidae, Graphium sarpedon, new subspecies. Zusammenfassung Die Beziehungen zwischen den Unterarten von Graphium sarpedon (Linnaeus, 1758) und anderer nahestehender Arten der Gattung werden untersucht. Diese Gruppe nahe verwandter Arten enthält wahrscheinlich acht Taxa wie folgt: Graphium sarpedon (Linnaeus, 1758), G. -

Two Projects of Butterfly Farming in Cambodia and Tanzania (Insecta: Lepidoptera) SHILAP Revista De Lepidopterología, Vol
SHILAP Revista de Lepidopterología ISSN: 0300-5267 [email protected] Sociedad Hispano-Luso-Americana de Lepidopterología España van der Heyden, T. Local and effective: Two projects of butterfly farming in Cambodia and Tanzania (Insecta: Lepidoptera) SHILAP Revista de Lepidopterología, vol. 39, núm. 155, septiembre, 2011, pp. 267-270 Sociedad Hispano-Luso-Americana de Lepidopterología Madrid, España Available in: http://www.redalyc.org/articulo.oa?id=45522101004 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative 267-270 Local and effective Tw 10/9/11 17:37 Página 267 SHILAP Revta. lepid., 39 (155), septiembre 2011: 267-270 CODEN: SRLPEF ISSN:0300-5267 Local and effective: Two projects of butterfly farming in Cambodia and Tanzania (Insecta: Lepidoptera) T. van der Heyden Abstract The projects “Banteay Srey Butterfly Centre” in Cambodia (Asia) and “Zanzibar Butterfly Centre” in Tanzania (Africa) are presented as models of sustainable butterfly farming to support local communities. KEY WORDS: Insecta, Lepidoptera, butterfly farming, sustainability, conservation, development, tropics, Cambodia, Tanzania. Local y efectivo: Dos proyectos de cría de mariposas en Camboya y Tanzania (Insecta: Lepidoptera) Resumen Los proyectos “Banteay Srey Butterfly Centre” en Camboya (Asia) y “Zanzibar Butterfly Centre” in Tanzania -

Pleistocene) Insect Assemblages from Illinois Kristine D
University of North Dakota UND Scholarly Commons Theses and Dissertations Theses, Dissertations, and Senior Projects 1985 Middle and Late Wisconsinan (Pleistocene) insect assemblages from Illinois Kristine D. Carter University of North Dakota Follow this and additional works at: https://commons.und.edu/theses Part of the Geology Commons Recommended Citation Carter, Kristine D., "Middle and Late Wisconsinan (Pleistocene) insect assemblages from Illinois" (1985). Theses and Dissertations. 52. https://commons.und.edu/theses/52 This Thesis is brought to you for free and open access by the Theses, Dissertations, and Senior Projects at UND Scholarly Commons. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of UND Scholarly Commons. For more information, please contact [email protected]. MIDDLE AND LATE WISCONSINAN (PLEISTOCENE) INSECT ASSEMBLAGES FROM ILLINOIS by Kristine D. Carter Bachelor of Science, North Dakota State University, 1981 B~chelor of Arts, Moorhead State University, 1978 A thesis submitted to the graduate faculty of the University of North Dakota in partial fulfillment of the requirements for the degree of Master of Arts Grand Forks, North Dakota May 1985 I" This thesis submitted by Kristine D. Carter in partial fulfillment of the requirements for the degree of Master of Arts from the University of North Dakota is hereby approved by the Faculty Advisory Committee under whom the work was done. This thesis meets the standards for appearance and conforms to the style and format requirements of the Graduate School of the University of North Dakota, and is hereby approved. Dean the Graduate School 55297:1 l. -

1968 a TAXONOMIC LIST of PHILATELIC LEPIDOPTERA Many
1968 Journal of the Lepidopterists' Society 241 A TAXONOMIC LIST OF PHILATELIC LEPIDOPTERA SIDNEY A. HESSEL Peabody Museum of Natural History, Yale University, New Haven, Conn. Many lepidopterists are also philatelists. This includes professional entomologists, some of whom are those actually responsible as insti gators or consultants for the many butterfly and moth postage stamps that have of late years appeared around the world. The first philatelic lepidopteran was issued in 1890 as an ornament in the hair of Hawaiian Queen Liliuokalani. Although one may speculate that it is the beautiful Vanessa tameamea Esch., it was not until 1930 when Lebanon honored the silk industry that a definitely determinable species was depicted. Stylized figures had appeared in the interval. In these instances the insects were, of course, incidental. Sarawak in 1950 was the first with nomenclature, Troides brookiana Wallace, which was figured unicolorous gray. It remained for the Swiss Pro Juventute issue of 1950 to honor the insect exclusively and in full color. This was largely the work of Dr. Loeliger, a member of our Society until his death and an important force in the Pro Juventute youth movement. The issue was accompanied by a brochure about the insects and was a most noteworthy effort towards stimulation of interest in Lepidoptera in that country. From this beginning, at first slowly, but with accelerated frequency, over 65 countries have "honored" species of Lepidoptera by 310 butterfly and 115 moth stamps, a total of 425 major varieties by the end of 1966. These embrace 248 species divided 181 and 67 respectively between butterflies and moths.