The Biodiversity of Flying Coleoptera Associated With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Checklist of the Coleoptera of New Brunswick, Canada
A peer-reviewed open-access journal ZooKeys 573: 387–512 (2016)Checklist of the Coleoptera of New Brunswick, Canada 387 doi: 10.3897/zookeys.573.8022 CHECKLIST http://zookeys.pensoft.net Launched to accelerate biodiversity research Checklist of the Coleoptera of New Brunswick, Canada Reginald P. Webster1 1 24 Mill Stream Drive, Charters Settlement, NB, Canada E3C 1X1 Corresponding author: Reginald P. Webster ([email protected]) Academic editor: P. Bouchard | Received 3 February 2016 | Accepted 29 February 2016 | Published 24 March 2016 http://zoobank.org/34473062-17C2-4122-8109-3F4D47BB5699 Citation: Webster RP (2016) Checklist of the Coleoptera of New Brunswick, Canada. In: Webster RP, Bouchard P, Klimaszewski J (Eds) The Coleoptera of New Brunswick and Canada: providing baseline biodiversity and natural history data. ZooKeys 573: 387–512. doi: 10.3897/zookeys.573.8022 Abstract All 3,062 species of Coleoptera from 92 families known to occur in New Brunswick, Canada, are re- corded, along with their author(s) and year of publication using the most recent classification framework. Adventive and Holarctic species are indicated. There are 366 adventive species in the province, 12.0% of the total fauna. Keywords Checklist, Coleoptera, New Brunswick, Canada Introduction The first checklist of the beetles of Canada by Bousquet (1991) listed 1,365 species from the province of New Brunswick, Canada. Since that publication, many species have been added to the faunal list of the province, primarily from increased collection efforts and -

Green-Tree Retention and Controlled Burning in Restoration and Conservation of Beetle Diversity in Boreal Forests
Dissertationes Forestales 21 Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Esko Hyvärinen Faculty of Forestry University of Joensuu Academic dissertation To be presented, with the permission of the Faculty of Forestry of the University of Joensuu, for public criticism in auditorium C2 of the University of Joensuu, Yliopistonkatu 4, Joensuu, on 9th June 2006, at 12 o’clock noon. 2 Title: Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Author: Esko Hyvärinen Dissertationes Forestales 21 Supervisors: Prof. Jari Kouki, Faculty of Forestry, University of Joensuu, Finland Docent Petri Martikainen, Faculty of Forestry, University of Joensuu, Finland Pre-examiners: Docent Jyrki Muona, Finnish Museum of Natural History, Zoological Museum, University of Helsinki, Helsinki, Finland Docent Tomas Roslin, Department of Biological and Environmental Sciences, Division of Population Biology, University of Helsinki, Helsinki, Finland Opponent: Prof. Bengt Gunnar Jonsson, Department of Natural Sciences, Mid Sweden University, Sundsvall, Sweden ISSN 1795-7389 ISBN-13: 978-951-651-130-9 (PDF) ISBN-10: 951-651-130-9 (PDF) Paper copy printed: Joensuun yliopistopaino, 2006 Publishers: The Finnish Society of Forest Science Finnish Forest Research Institute Faculty of Agriculture and Forestry of the University of Helsinki Faculty of Forestry of the University of Joensuu Editorial Office: The Finnish Society of Forest Science Unioninkatu 40A, 00170 Helsinki, Finland http://www.metla.fi/dissertationes 3 Hyvärinen, Esko 2006. Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests. University of Joensuu, Faculty of Forestry. ABSTRACT The main aim of this thesis was to demonstrate the effects of green-tree retention and controlled burning on beetles (Coleoptera) in order to provide information applicable to the restoration and conservation of beetle species diversity in boreal forests. -

Insecta: Coleoptera: Leiodidae: Cholevinae), with a Description of Sciaphyes Shestakovi Sp.N
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arthropod Systematics and Phylogeny Jahr/Year: 2011 Band/Volume: 69 Autor(en)/Author(s): Fresneda Javier, Grebennikov Vasily V., Ribera Ignacio Artikel/Article: The phylogenetic and geographic limits of Leptodirini (Insecta: Coleoptera: Leiodidae: Cholevinae), with a description of Sciaphyes shestakovi sp.n. from the Russian Far East 99-123 Arthropod Systematics & Phylogeny 99 69 (2) 99 –123 © Museum für Tierkunde Dresden, eISSN 1864-8312, 21.07.2011 The phylogenetic and geographic limits of Leptodirini (Insecta: Coleoptera: Leiodidae: Cholevinae), with a description of Sciaphyes shestakovi sp. n. from the Russian Far East JAVIER FRESNEDA 1, 2, VASILY V. GREBENNIKOV 3 & IGNACIO RIBERA 4, * 1 Ca de Massa, 25526 Llesp, Lleida, Spain 2 Museu de Ciències Naturals (Zoologia), Passeig Picasso s/n, 08003 Barcelona, Spain [[email protected]] 3 Ottawa Plant Laboratory, Canadian Food Inspection Agency, 960 Carling Avenue, Ottawa, Ontario, K1A 0C6, Canada [[email protected]] 4 Institut de Biologia Evolutiva (CSIC-UPF), Passeig Marítim de la Barceloneta, 37 – 49, 08003 Barcelona, Spain [[email protected]] * Corresponding author Received 26.iv.2011, accepted 27.v.2011. Published online at www.arthropod-systematics.de on 21.vii.2011. > Abstract The tribe Leptodirini of the beetle family Leiodidae is one of the most diverse radiations of cave animals, with a distribution centred north of the Mediterranean basin from the Iberian Peninsula to Iran. Six genera outside this core area, most notably Platycholeus Horn, 1880 in the western United States and others in East Asia, have been assumed to be related to Lepto- dirini. -

Newsletter of the Biological Survey of Canada
Newsletter of the Biological Survey of Canada Vol. 40(1) Summer 2021 The Newsletter of the BSC is published twice a year by the In this issue Biological Survey of Canada, an incorporated not-for-profit From the editor’s desk............2 group devoted to promoting biodiversity science in Canada. Membership..........................3 President’s report...................4 BSC Facebook & Twitter...........5 Reminder: 2021 AGM Contributing to the BSC The Annual General Meeting will be held on June 23, 2021 Newsletter............................5 Reminder: 2021 AGM..............6 Request for specimens: ........6 Feature Articles: Student Corner 1. City Nature Challenge Bioblitz Shawn Abraham: New Student 2021-The view from 53.5 °N, Liaison for the BSC..........................7 by Greg Pohl......................14 Mayflies (mainlyHexagenia sp., Ephemeroptera: Ephemeridae): an 2. Arthropod Survey at Fort Ellice, MB important food source for adult by Robert E. Wrigley & colleagues walleye in NW Ontario lakes, by A. ................................................18 Ricker-Held & D.Beresford................8 Project Updates New book on Staphylinids published Student Corner by J. Klimaszewski & colleagues......11 New Student Liaison: Assessment of Chironomidae (Dip- Shawn Abraham .............................7 tera) of Far Northern Ontario by A. Namayandeh & D. Beresford.......11 Mayflies (mainlyHexagenia sp., Ephemerop- New Project tera: Ephemeridae): an important food source Help GloWorm document the distribu- for adult walleye in NW Ontario lakes, tion & status of native earthworms in by A. Ricker-Held & D.Beresford................8 Canada, by H.Proctor & colleagues...12 Feature Articles 1. City Nature Challenge Bioblitz Tales from the Field: Take me to the River, by Todd Lawton ............................26 2021-The view from 53.5 °N, by Greg Pohl..............................14 2. -

Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016
Old Woman Creek National Estuarine Research Reserve Management Plan 2011-2016 April 1981 Revised, May 1982 2nd revision, April 1983 3rd revision, December 1999 4th revision, May 2011 Prepared for U.S. Department of Commerce Ohio Department of Natural Resources National Oceanic and Atmospheric Administration Division of Wildlife Office of Ocean and Coastal Resource Management 2045 Morse Road, Bldg. G Estuarine Reserves Division Columbus, Ohio 1305 East West Highway 43229-6693 Silver Spring, MD 20910 This management plan has been developed in accordance with NOAA regulations, including all provisions for public involvement. It is consistent with the congressional intent of Section 315 of the Coastal Zone Management Act of 1972, as amended, and the provisions of the Ohio Coastal Management Program. OWC NERR Management Plan, 2011 - 2016 Acknowledgements This management plan was prepared by the staff and Advisory Council of the Old Woman Creek National Estuarine Research Reserve (OWC NERR), in collaboration with the Ohio Department of Natural Resources-Division of Wildlife. Participants in the planning process included: Manager, Frank Lopez; Research Coordinator, Dr. David Klarer; Coastal Training Program Coordinator, Heather Elmer; Education Coordinator, Ann Keefe; Education Specialist Phoebe Van Zoest; and Office Assistant, Gloria Pasterak. Other Reserve staff including Dick Boyer and Marje Bernhardt contributed their expertise to numerous planning meetings. The Reserve is grateful for the input and recommendations provided by members of the Old Woman Creek NERR Advisory Council. The Reserve is appreciative of the review, guidance, and council of Division of Wildlife Executive Administrator Dave Scott and the mapping expertise of Keith Lott and the late Steve Barry. -

Coleópteros Saproxílicos De Los Bosques De Montaña En El Norte De La Comunidad De Madrid
Universidad Politécnica de Madrid Escuela Técnica Superior de Ingenieros Agrónomos Coleópteros Saproxílicos de los Bosques de Montaña en el Norte de la Comunidad de Madrid T e s i s D o c t o r a l Juan Jesús de la Rosa Maldonado Licenciado en Ciencias Ambientales 2014 Departamento de Producción Vegetal: Botánica y Protección Vegetal Escuela Técnica Superior de Ingenieros Agrónomos Coleópteros Saproxílicos de los Bosques de Montaña en el Norte de la Comunidad de Madrid Juan Jesús de la Rosa Maldonado Licenciado en Ciencias Ambientales Directores: D. Pedro del Estal Padillo, Doctor Ingeniero Agrónomo D. Marcos Méndez Iglesias, Doctor en Biología 2014 Tribunal nombrado por el Magfco. y Excmo. Sr. Rector de la Universidad Politécnica de Madrid el día de de 2014. Presidente D. Vocal D. Vocal D. Vocal D. Secretario D. Suplente D. Suplente D. Realizada la lectura y defensa de la Tesis el día de de 2014 en Madrid, en la Escuela Técnica Superior de Ingenieros Agrónomos. Calificación: El Presidente Los Vocales El Secretario AGRADECIMIENTOS A Ángel Quirós, Diego Marín Armijos, Isabel López, Marga López, José Luis Gómez Grande, María José Morales, Alba López, Jorge Martínez Huelves, Miguel Corra, Adriana García, Natalia Rojas, Rafa Castro, Ana Busto, Enrique Gorroño y resto de amigos que puntualmente colaboraron en los trabajos de campo o de gabinete. A la Guardería Forestal de la comarca de Buitrago de Lozoya, por su permanente apoyo logístico. A los especialistas en taxonomía que participaron en la identificación del material recolectado, pues sin su asistencia hubiera sido mucho más difícil finalizar este trabajo. -

The Invertebrate Fauna of Dune and Machair Sites In
INSTITUTE OF TERRESTRIAL ECOLOGY (NATURAL ENVIRONMENT RESEARCH COUNCIL) REPORT TO THE NATURE CONSERVANCY COUNCIL ON THE INVERTEBRATE FAUNA OF DUNE AND MACHAIR SITES IN SCOTLAND Vol I Introduction, Methods and Analysis of Data (63 maps, 21 figures, 15 tables, 10 appendices) NCC/NE RC Contract No. F3/03/62 ITE Project No. 469 Monks Wood Experimental Station Abbots Ripton Huntingdon Cambs September 1979 This report is an official document prepared under contract between the Nature Conservancy Council and the Natural Environment Research Council. It should not be quoted without permission from both the Institute of Terrestrial Ecology and the Nature Conservancy Council. (i) Contents CAPTIONS FOR MAPS, TABLES, FIGURES AND ArPENDICES 1 INTRODUCTION 1 2 OBJECTIVES 2 3 METHODOLOGY 2 3.1 Invertebrate groups studied 3 3.2 Description of traps, siting and operating efficiency 4 3.3 Trapping period and number of collections 6 4 THE STATE OF KNOWL:DGE OF THE SCOTTISH SAND DUNE FAUNA AT THE BEGINNING OF THE SURVEY 7 5 SYNOPSIS OF WEATHER CONDITIONS DURING THE SAMPLING PERIODS 9 5.1 Outer Hebrides (1976) 9 5.2 North Coast (1976) 9 5.3 Moray Firth (1977) 10 5.4 East Coast (1976) 10 6. THE FAUNA AND ITS RANGE OF VARIATION 11 6.1 Introduction and methods of analysis 11 6.2 Ordinations of species/abundance data 11 G. Lepidoptera 12 6.4 Coleoptera:Carabidae 13 6.5 Coleoptera:Hydrophilidae to Scolytidae 14 6.6 Araneae 15 7 THE INDICATOR SPECIES ANALYSIS 17 7.1 Introduction 17 7.2 Lepidoptera 18 7.3 Coleoptera:Carabidae 19 7.4 Coleoptera:Hydrophilidae to Scolytidae -

Agathidium Vaderi MILLER & WHEELER, 2005
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Linzer biologische Beiträge Jahr/Year: 2021 Band/Volume: 0052_2 Autor(en)/Author(s): Schifko Georg Artikel/Article: Agathidium vaderi MILLER & WHEELER, 2005 (Coleoptera, Leiodidae) – Ein wissenschaftlicher Artname im Spannungsfeld der Zoologie, der Ethnologie und der Populärkultur 1099-1104 Linzer biol. Beitr. 52/2 1099-1104 Februar 2021 Agathidium vaderi MILLER & WHEELER, 2005 (Coleoptera, Leiodidae) – Ein wissenschaftlicher Artname im Spannungsfeld der Zoologie, der Ethnologie und der Populärkultur Georg SCHIFKO A b s t r a c t : In recent times, names related to popular culture have increasingly been given to new discovered species. This article deals with the Agathidium vaderi beetle named in 2005 by Kelly B. Miller and Quentin D. Wheeler after the fictional character Darth Vader due to the postulated similarity of the beetle's head with Darth Vader's helmet which is ultimately based on Japanese Samurai helmet designs. Additionally, attention is drawn to the the mutual benefits available to the taxonomy as well as for the films in the Star Wars series due to the unconventual species name. Key Words: Agathidium vaderi, Star Wars, Japanese helmets, Taxonomy, Nomenclature Einleitung Im Japanischen wird der zur Familie der Blatthornkäfer (Scarabaeidae) gehörende Allomyrina dichotoma (LINNAEUS, 1771) als kabutomushi bezeichnet, was soviel wie "Helminsekt" (kabuto = Helm, mushi = Insekt) bedeutet und eine Anspielung auf japanische Samuraihelme darstellt. A. dichotoma bildet im Land der aufgehenden Sonne einen fest verankerten Bestandteil der Populärkultur und man lässt dort Männchen dieser Spezies gegeneinander kämpfen. In den Wettbewerben geht es darum, wessen Käfer die schwerste Last ziehen kann und um Spiele, bei denen man die Käfermännchen dazu bringt um ein Stück Wassermelone zu kämpfen (LAURENT 2001: 70) – somit "eine Insekten- version des Sumo-Ringens" (HERZOG 2012: 57). -

A Comparison of Three Trapping Methods Used to Survey Forest-Dwelling Coleoptera
Eur. J. Entomol. 103: 397–407, 2006 ISSN 1210-5759 A comparison of three trapping methods used to survey forest-dwelling Coleoptera ESKO HYVÄRINEN, JARI KOUKI and PETRI MARTIKAINEN Faculty of Forest Sciences, University of Joensuu, P.O. Box 111, FIN – 80101 Joensuu, Finland; e-mail: [email protected] Keywords. Abundance distribution, beetles, biodiversity inventories, boreal forest, Coleoptera, interception traps, pitfall traps, sampling, species richness, window traps Abstract. Sampling of insect communities is very challenging and for reliable interpretation of results the effects of different sam- pling protocols and data processing on the results need to be fully understood. We compared three different commonly used methods for sampling forest beetles, freely hanging flight-intercept (window) traps (FWT), flight-intercept traps attached to trunks (TWT) and pitfall traps placed in the ground (PFT), in Scots pine dominated boreal forests in eastern Finland. Using altogether 960 traps, forming 576 sub-samples, at 24 study sites, 59760 beetles belonging to 814 species were collected over a period of a month. All of the material was identified to species, with the exception of a few species pairs, to obtain representative data for analyses. Four partly overlapping groups were used in the analyses: (1) all, (2) saproxylic, (3) rare and (4) red-listed species. In terms of the number of species collected TWTs were the most effective for all species groups and the rarer species the species group composed of (groups 1-2-3-4) the larger were the differences between the trap types. In particular, the TWTs caught most red-listed species. However, when sample sizes were standardized FWTs and TWTs caught similar number of species of all species groups. -

293 315 Nikitskij.P65
Åâðàçèàòñêèé ýíòîìîë. æóðíàë 4(4): 293315 © EUROASIAN ENTOMOLOGICAL JOURNAL, 2005 Ñèñòåìàòè÷åñêèé ñïèñîê æåñòêîêðûëûõ (Insecta, Coleoptera) Óäìóðòèè Checklist of beetles (Insecta, Coleoptera) of Udmurtia Ñ.Â. Äåäþõèí*, Í.Á. Íèêèòñêèé**, Â.Á. Ñåì¸íîâ*** S.V. Dedyukhin*, N.B. Nikitsky**, V.B. Semenov*** * Êàôåäðà ýêîëîãèè æèâîòíûõ, Óäìóðòñêèé ãîñóäàðñòâåííûé óíèâåðñèòåò, óë. Óíèâåðñèòåòñêàÿ 1, Èæåâñê 426034 Ðîññèÿ. E-mail: [email protected]. * Department of animal ecology, Udmurt State University, Universitetskaya str. 1, Izhevsk 426034 Russia. ** Çîîëîãè÷åñêèé ìóçåé ÌÃÓ èì. Ì.Â. Ëîìîíîñîâà, óë. Áîëüøàÿ Íèêèòñêàÿ 6, Ìîñêâà 125009 Ðîññèÿ. Email: [email protected]. ** Zoological Museum of Moscow Lomonosov State University, Bolshaya Nikitskaya 6, Moscow 125009 Russia. *** Èíñòèòóò ìåäèöèíñêîé ïàðàçèòîëîãèè è òðîïè÷åñêîé ìåäèöèíû èì. Å.È. Ìàðöèíîâñêîãî, óë. Ìàëàÿ Ïèðîãîâñêàÿ 20, Ìîñêâà 119435 Ðîññèÿ. *** Martsinovsky Institute of Medical Parasitology and Tropical Medicine, Malaya Pirogovskaya 20, Moscow 119435 Russia. Êëþ÷åâûå ñëîâà: Coleoptera, ôàóíà, Óäìóðòèÿ. Key words: Coleoptera, fauna, Udmurtia. Ðåçþìå. Ïðèâîäèòñÿ ïîëíûé ñïèñîê æåñòêîêðûëûõ óêàçàííûå â ïåðâîì ñïèñêå, çäåñü îòñóòñòâóþò, ÷òî, íàñåêîìûõ (Coleoptera) Óäìóðòèè, ãäå îòìå÷åíî íåìíî- ïî-âèäèìîìó, ãîâîðèò îá èõ îøèáî÷íîì îïðåäåëåíèè. ãèì áîëåå 2400 âèäîâ æóêîâ èç 91 ñåìåéñòâà. Âïåðâûå Ñ íàèáîëüøåé (ïðîïîðöèîíàëüíî îáùåìó ïîòåíöèàëü- äëÿ ðåãèîíà ïðèâåäåíî 1482 âèäà (ïîìå÷åíû â ñïèñêå íîìó ÷èñëó âèäîâ) ïîëíîòîé áûëè èçó÷åíû ñåìåéñòâà: çâåçäî÷êîé *, ñ ïðàâîé ñòîðîíû îò íàçâàíèÿ âèäà). Carabidae (139 âèäîâ), Cerambycidae (58), Scarabae- Abstract. A complete checklist (2400 species in 91 idae (s. l.) (57 âèäîâ), Elateridae (50 âèäîâ), à òàêæå families) of the beetles (Coleoptera) of Udmurtia is provided. Curculionidae (s. l.) (76), Buprestidae (20), Coccinellidae 1482 species are reported from Udmurtia for the first time, (17), äëÿ îñòàëüíûõ ïðèâåäåíû ëèøü îòäåëüíûå ïðåä- as indicated by an asterisk (*) in the list. -

Laboratory Methods for Rearing Soil Beetles (Coleoptera)
ZOOLOGICA Bolesław Burakowski Laboratory methods for rearing soil beetles (Coleoptera) Polska Akademia Nauk Muzeum i Instytut Zoologii Warszawa 1993 http://rcin.org.pl POLSKA AKADEMIA NAUK MUZEUM I INSTYTUT ZOOLOGII MEMORABILIA ZOOLOGICA 46 Bolesław Burakowski Laboratory methods for rearing soil beetles (Coleopter a) WARSZAWA 1993 http://rcin.org.pl MEMORABILIA ZOOLOGICA, 46, 1993 World-list abbreviation: Memorabilia Zool. EDITORIAL STAFF Editor — in — chief — Bohdan Pisarski Asistant editor — Wojciech Czechowski Secretary — Katarzyna Cholewicka-Wiśniewska Editor of the volume — Wojciech Czechowski Publisher Muzeum i Instytut Zoologii PAN ul. Wilcza 64, 00-679 Warszawa PL ISSN 0076-6372 ISBN 83-85192-12-3 © Copyright by Muzeum i Instytut Zoologii PAN Warszawa 1993 Nakład 1000 egz. Ark. wyd. 5,5. Ark. druk 4 Druk: Zakład Poligraficzno-Wydawniczy „StangraF’ http://rcin.org.pl Bolesław Bu r a k o w sk i Laboratory methods for rearing soil beetles ( Coleoptera) INTRODUCTION Beetles are the most numerous group of insects; nearly 300,000 species have been described up till now, and about 6,000 of these occur in Poland. The morphological variability and different modes of life result from beetle ability to adapt to all kinds of habitats. Terrestrial and soil living forms dominate. Beetles undergo a complete metamorphosis and most species live in soil during at least one of the stages. They include predators, herbivores, parasites and sapro- phagans, playing a fairly significant role in nature and in man’s economy. Our knowledge of beetles, even of the common species, is insufficient. In spite of the fact that the beetle fauna of Central Europe has been studied relatively well, the knowledge accumulated is generally limited to the adults, while the immature stages have not been adequately studied. -
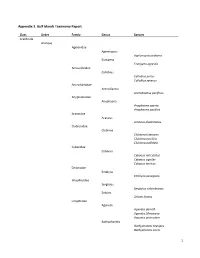
1 Appendix 3. Gulf Islands Taxonomy Report
Appendix 3. Gulf Islands Taxonomy Report Class Order Family Genus Species Arachnida Araneae Agelenidae Agelenopsis Agelenopsis utahana Eratigena Eratigena agrestis Amaurobiidae Callobius Callobius pictus Callobius severus Antrodiaetidae Antrodiaetus Antrodiaetus pacificus Anyphaenidae Anyphaena Anyphaena aperta Anyphaena pacifica Araneidae Araneus Araneus diadematus Clubionidae Clubiona Clubiona lutescens Clubiona pacifica Clubiona pallidula Cybaeidae Cybaeus Cybaeus reticulatus Cybaeus signifer Cybaeus tetricus Dictynidae Emblyna Emblyna peragrata Gnaphosidae Sergiolus Sergiolus columbianus Zelotes Zelotes fratris Linyphiidae Agyneta Agyneta darrelli Agyneta fillmorana Agyneta protrudens Bathyphantes Bathyphantes brevipes Bathyphantes keeni 1 Centromerita Centromerita bicolor Ceratinops Ceratinops latus Entelecara Entelecara acuminata Erigone Erigone aletris Erigone arctica Erigone cristatopalpus Frederickus Frederickus coylei Grammonota Grammonota kincaidi Linyphantes Linyphantes nehalem Linyphantes nigrescens Linyphantes pacificus Linyphantes pualla Linyphantes victoria Mermessus Mermessus trilobatus Microlinyphia Microlinyphia dana Neriene Neriene digna Neriene litigiosa Oedothorax Oedothorax alascensis Pityohyphantes Pityohyphantes alticeps Pocadicnemis Pocadicnemis pumila Poeciloneta Poeciloneta fructuosa Saaristoa Saaristoa sammamish Scotinotylus Scotinotylus sp. 5GAB Semljicola Semljicola sp. 1GAB Sisicottus Spirembolus Spirembolus abnormis Spirembolus mundus Tachygyna Tachygyna ursina Tachygyna vancouverana Tapinocyba Tapinocyba