Tilbury Power Station Essex, Invertebrate Survey Report (June 2008)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
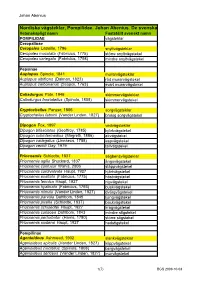
Nordiska Vägsteklar, Pompilidae. Johan Abenius. De Svenska
Johan Abenius Nordiska vägsteklar, Pompilidae. Johan Abenius. De svenska namnen fastställda av Kommittén för svenska djurnamn 100825 Vetenskapligt namn Fastställt svenskt namn POMPILIDAE vägsteklar Ceropalinae Ceropales Latreille, 1796 snyltvägsteklar Ceropales maculata (Fabricius, 1775) större snyltvägstekel Ceropales variegata (Fabricius, 1798) mindre snyltvägstekel Pepsinae Auplopus Spinola, 1841 murarvägsteklar Auplopus albifrons (Dalman, 1823) röd murarvägstekel Auplopus carbonarius (Scopoli, 1763) svart murarvägstekel Caliadurgus Pate, 1946 skimmervägsteklar Caliadurgus fasciatellus (Spinola, 1808) skimmervägstekel Cryptocheilus Panzer, 1806 sorgvägsteklar Cryptocheilus fabricii (Vander Linden, 1827) brokig sorgvägstekel Dipogon Fox, 1897 vedvägsteklar Dipogon bifasciatus (Geoffroy, 1785) björkvägstekel Dipogon subintermedius (Magretti, 1886) ekvägstekel Dipogon variegatus (Linnaeus, 1758) aspvägstekel Dipogon vechti Day, 1979 tallvägstekel Priocnemis Schiødte, 1837 sågbensvägsteklar Priocnemis agilis Shuckard, 1837 ängsvägstekel Priocnemis confusor Wahis, 2006 stäppvägstekel Priocnemis cordivalvata Haupt, 1927 hjärtvägstekel Priocnemis exaltata (Fabricius, 1775) höstvägstekel Priocnemis fennica Haupt, 1927 nipvägstekel Priocnemis hyalinata (Fabricius, 1793) buskvägstekel Priocnemis minuta (Vander Linden, 1827) dvärgvägstekel Priocnemis parvula Dahlbom, 1845 ljungvägstekel Priocnemis pusilla (Schiødte, 1837) backvägstekel Priocnemis schioedtei Haupt, 1927 kragvägstekel Priocnemis coriacea Dahlbom, 1843 mindre stigstekel Priocnemis -

Green-Tree Retention and Controlled Burning in Restoration and Conservation of Beetle Diversity in Boreal Forests
Dissertationes Forestales 21 Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Esko Hyvärinen Faculty of Forestry University of Joensuu Academic dissertation To be presented, with the permission of the Faculty of Forestry of the University of Joensuu, for public criticism in auditorium C2 of the University of Joensuu, Yliopistonkatu 4, Joensuu, on 9th June 2006, at 12 o’clock noon. 2 Title: Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests Author: Esko Hyvärinen Dissertationes Forestales 21 Supervisors: Prof. Jari Kouki, Faculty of Forestry, University of Joensuu, Finland Docent Petri Martikainen, Faculty of Forestry, University of Joensuu, Finland Pre-examiners: Docent Jyrki Muona, Finnish Museum of Natural History, Zoological Museum, University of Helsinki, Helsinki, Finland Docent Tomas Roslin, Department of Biological and Environmental Sciences, Division of Population Biology, University of Helsinki, Helsinki, Finland Opponent: Prof. Bengt Gunnar Jonsson, Department of Natural Sciences, Mid Sweden University, Sundsvall, Sweden ISSN 1795-7389 ISBN-13: 978-951-651-130-9 (PDF) ISBN-10: 951-651-130-9 (PDF) Paper copy printed: Joensuun yliopistopaino, 2006 Publishers: The Finnish Society of Forest Science Finnish Forest Research Institute Faculty of Agriculture and Forestry of the University of Helsinki Faculty of Forestry of the University of Joensuu Editorial Office: The Finnish Society of Forest Science Unioninkatu 40A, 00170 Helsinki, Finland http://www.metla.fi/dissertationes 3 Hyvärinen, Esko 2006. Green-tree retention and controlled burning in restoration and conservation of beetle diversity in boreal forests. University of Joensuu, Faculty of Forestry. ABSTRACT The main aim of this thesis was to demonstrate the effects of green-tree retention and controlled burning on beetles (Coleoptera) in order to provide information applicable to the restoration and conservation of beetle species diversity in boreal forests. -

Dipterists Digest
Dipterists Digest 2019 Vol. 26 No. 1 Cover illustration: Eliozeta pellucens (Fallén, 1820), male (Tachinidae) . PORTUGAL: Póvoa Dão, Silgueiros, Viseu, N 40º 32' 59.81" / W 7º 56' 39.00", 10 June 2011, leg. Jorge Almeida (photo by Chris Raper). The first British record of this species is reported in the article by Ivan Perry (pp. 61-62). Dipterists Digest Vol. 26 No. 1 Second Series 2019 th Published 28 June 2019 Published by ISSN 0953-7260 Dipterists Digest Editor Peter J. Chandler, 606B Berryfield Lane, Melksham, Wilts SN12 6EL (E-mail: [email protected]) Editorial Panel Graham Rotheray Keith Snow Alan Stubbs Derek Whiteley Phil Withers Dipterists Digest is the journal of the Dipterists Forum . It is intended for amateur, semi- professional and professional field dipterists with interests in British and European flies. All notes and papers submitted to Dipterists Digest are refereed. Articles and notes for publication should be sent to the Editor at the above address, and should be submitted with a current postal and/or e-mail address, which the author agrees will be published with their paper. Articles must not have been accepted for publication elsewhere and should be written in clear and concise English. Contributions should be supplied either as E-mail attachments or on CD in Word or compatible formats. The scope of Dipterists Digest is: - the behaviour, ecology and natural history of flies; - new and improved techniques (e.g. collecting, rearing etc.); - the conservation of flies; - reports from the Diptera Recording Schemes, including maps; - records and assessments of rare or scarce species and those new to regions, countries etc.; - local faunal accounts and field meeting results, especially if accompanied by ecological or natural history interpretation; - descriptions of species new to science; - notes on identification and deletions or amendments to standard key works and checklists. -

Insecta: Coleoptera: Leiodidae: Cholevinae), with a Description of Sciaphyes Shestakovi Sp.N
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Arthropod Systematics and Phylogeny Jahr/Year: 2011 Band/Volume: 69 Autor(en)/Author(s): Fresneda Javier, Grebennikov Vasily V., Ribera Ignacio Artikel/Article: The phylogenetic and geographic limits of Leptodirini (Insecta: Coleoptera: Leiodidae: Cholevinae), with a description of Sciaphyes shestakovi sp.n. from the Russian Far East 99-123 Arthropod Systematics & Phylogeny 99 69 (2) 99 –123 © Museum für Tierkunde Dresden, eISSN 1864-8312, 21.07.2011 The phylogenetic and geographic limits of Leptodirini (Insecta: Coleoptera: Leiodidae: Cholevinae), with a description of Sciaphyes shestakovi sp. n. from the Russian Far East JAVIER FRESNEDA 1, 2, VASILY V. GREBENNIKOV 3 & IGNACIO RIBERA 4, * 1 Ca de Massa, 25526 Llesp, Lleida, Spain 2 Museu de Ciències Naturals (Zoologia), Passeig Picasso s/n, 08003 Barcelona, Spain [[email protected]] 3 Ottawa Plant Laboratory, Canadian Food Inspection Agency, 960 Carling Avenue, Ottawa, Ontario, K1A 0C6, Canada [[email protected]] 4 Institut de Biologia Evolutiva (CSIC-UPF), Passeig Marítim de la Barceloneta, 37 – 49, 08003 Barcelona, Spain [[email protected]] * Corresponding author Received 26.iv.2011, accepted 27.v.2011. Published online at www.arthropod-systematics.de on 21.vii.2011. > Abstract The tribe Leptodirini of the beetle family Leiodidae is one of the most diverse radiations of cave animals, with a distribution centred north of the Mediterranean basin from the Iberian Peninsula to Iran. Six genera outside this core area, most notably Platycholeus Horn, 1880 in the western United States and others in East Asia, have been assumed to be related to Lepto- dirini. -

The Magazine of the British Dragonfly Society Spring 2013 Favourite Days 30Th Anniversary Stamp Issue
Dragonfly 63 NewsThe Magazine of the British Dragonfly Society Spring 2013 www.british-dragonflies.org.uk Favourite Days 30th Anniversary stamp issue Observations On the Trail of the Orange-spotted Emerald Dragonfly News 63 The Magazine of the British Dragonfly Society Published twice a year, in April and October, Dragonfly News covers all aspects of the British Dragonfly Society’s field, recording, monitoring, research, conservation and social activities, as well as information from the wider dragonfly, natural history and conservation world. The emphasis is on dragonflies recorded in the UK. *The British Dragonfly Society aims to promote and encourage the study, conservation and understanding of dragonflies and their natural habitats, especially in the UK, and to raise public awareness of dragonflies. Dragonfly News is edited & designed by: Trustees & Officers of the BDS Mark Tyrrell, 8 Warwick Close, Raunds, Chairman: Pam Taylor, Decoy Farm, Decoy Road, Potter Northants., NN9 6JH Tel. Heigham, Norfolk, NR29 5LX. Tel. e-mail: Vice-Chairman: Vacant Deadlines for inclusion of copy: Secretary: Henry Curry, 23 Bowker Way, Whittlesey, Spring 31 January Peterborough, PE7 1PY. Tel. Autumn 31 July Treasurer: Brian Walker, 49 Roman Way, Wantage, Advertising Rates: Oxfordshire, OX12 9YF. Tel. £15 for small-ad (text only); £40 for quarter- Trustees: Andy Harmer, Alan Nelson, *Mick Parfitt. page; £60 for half-page; £100 for full-page. Journal Editor: Peter Mill, 8 Cookridge Grove, LEEDS, LS16 7LH. © British Dragonfly Society 2013 Shop Manager: Lynn Curry, 23 Bowker Way, Whittlesey, All rights reserved. No part of this publication may be Peterborough, PE7 1PY Tel. reproduced, stored in a retrieval system or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the permission of the British Dragonfly Conservation Group (DCG) Dragonfly Society or the copyright owner. -

Nota Lepidopterologica
©Societas Europaea Lepidopterologica; download unter http://www.biodiversitylibrary.org/ und www.zobodat.at Nota lepid. 22 (3): 212-226; 01.IX.1999 ISSN 0342-7536 Notes on some Western Palaearctic species of Bucculatrix (Gracillarioidea, Bucculatricidae) Wolfram Mey Museum für Naturkunde, Humboldt-Universität Berlin, Invalidenstraße 43, D-101 15 Berlin Summary. The type material of 12 species of Bucculatrix Zeller, 1839 deposited in the Museum für Naturkunde Berlin is revised. B. imitatella Herrich-Schäffer, [1855], and B. jugicola Wocke, 1877, are sunk in synonymy of B. cristatella (Zeller, 1839). Two other synonyms have been established: B. alpina Frey, 1870 = B. leucanthemella Constant, 1895, syn. n.; B. infans Staudinger, 1880 = B. centaureae Deschka, 1973, syn. n. The male genitalia of the species are figured. Lectotypes have been designated for 5 species. Zusammenfassung. Es wird das Typenmaterial von 12 Arten der Gattung Bucculatrix Zeller, 1839 revidiert, die sich im Museum für Naturkunde Berlin befinden. Zwei Namen stellten sich als neue Synonyme heraus: B. imitatella Herrich-Schäffer, [1855], syn. n. und B. jugicola Wocke, 1877, syn. n. von B. cristatella (Zeller, 1839). Zwei weitere Synonyme werden bekanntgemacht: B. leucanthemella Constant, 1895, syn. n. von B. alpina Frey, 1870 und B. centaureae Deschka, 1973, syn. n. von B. infans Staudinger, 1880. Für fünf Arten werden Lectotypen festgelegt. Résumé. Le matériel-type de 12 espèces du genre Bucculatrix Zeller, 1839, déposé au Museum für Naturkunde Berlin, a été révisé. Deux noms sont apparus comme étant de nouveaux synonymes: B. imitatella Herrich-Schäffer, [1855], syn. n. et B. jugicola Wocke, 1877, syn. n. de B. cristatella (Zeller, 1839). -

The Tiger Beetles (Coleoptera, Cicindelidae) of the Southern Levant and Adjacent Territories: from Cybertaxonomy to Conservation Biology
A peer-reviewed open-access journal ZooKeys 734: 43–103 The(2018) tiger beetles( Coleoptera, Cicindelidae) of the southern Levant... 43 doi: 10.3897/zookeys.734.21989 MONOGRAPH http://zookeys.pensoft.net Launched to accelerate biodiversity research The tiger beetles (Coleoptera, Cicindelidae) of the southern Levant and adjacent territories: from cybertaxonomy to conservation biology Thorsten Assmann1, Estève Boutaud1, Jörn Buse2, Jörg Gebert3, Claudia Drees4,5, Ariel-Leib-Leonid Friedman4, Fares Khoury6, Tamar Marcus1, Eylon Orbach7, Ittai Renan4, Constantin Schmidt8, Pascale Zumstein1 1 Institute of Ecology, Leuphana University Lüneburg, Universitätsallee 1, D-21335 Lüneburg, Germany 2 Ecosystem Monitoring, Research and Wildlife Conservation (SB 23 Invertebrates and Biodiversity), Black Forest National Park, Kniebisstraße 67, D-72250 Freudenstadt, Germany 3 Karl-Liebknecht-Straße 73, D-01109 Dresden. Germany 4 Steinhardt Museum of Natural History, Tel Aviv University, Ramat-Aviv, Tel Aviv, IL-69978, Israel 5 Biocentre Grindel, Universität Hamburg, Martin-Luther-King-Platz 3, D-20146 Hamburg, Germany 6 Department of Biology and Biotechnology, American University of Madaba, P.O.Box 2882, Amman, JO-11821, Jordan 7 Remez St. 49, IL-36044 Qiryat Tiv’on, Israel 8 Deichstr. 13, D-21354 Bleckede, Germany Corresponding author: Thorsten Assmann ([email protected]) Academic editor: B. Guéorguiev | Received 1 November 2017 | Accepted 15 January 2018 | Published 5 February 2018 http://zoobank.org/7C3C687B-64BB-42A5-B9E4-EC588BCD52D5 Citation: Assmann T, Boutaud E, Buse J, Gebert J, Drees C, Friedman A-L-L, Khoury F, Marcus T, Orbach E, Renan I, Schmidt C, Zumstein P (2018) The tiger beetles (Coleoptera, Cicindelidae) of the southern Levant and adjacent territories: from cybertaxonomy to conservation biology. -

Final Report 1
Sand pit for Biodiversity at Cep II quarry Researcher: Klára Řehounková Research group: Petr Bogusch, David Boukal, Milan Boukal, Lukáš Čížek, František Grycz, Petr Hesoun, Kamila Lencová, Anna Lepšová, Jan Máca, Pavel Marhoul, Klára Řehounková, Jiří Řehounek, Lenka Schmidtmayerová, Robert Tropek Březen – září 2012 Abstract We compared the effect of restoration status (technical reclamation, spontaneous succession, disturbed succession) on the communities of vascular plants and assemblages of arthropods in CEP II sand pit (T řebo ňsko region, SW part of the Czech Republic) to evaluate their biodiversity and conservation potential. We also studied the experimental restoration of psammophytic grasslands to compare the impact of two near-natural restoration methods (spontaneous and assisted succession) to establishment of target species. The sand pit comprises stages of 2 to 30 years since site abandonment with moisture gradient from wet to dry habitats. In all studied groups, i.e. vascular pants and arthropods, open spontaneously revegetated sites continuously disturbed by intensive recreation activities hosted the largest proportion of target and endangered species which occurred less in the more closed spontaneously revegetated sites and which were nearly absent in technically reclaimed sites. Out results provide clear evidence that the mosaics of spontaneously established forests habitats and open sand habitats are the most valuable stands from the conservation point of view. It has been documented that no expensive technical reclamations are needed to restore post-mining sites which can serve as secondary habitats for many endangered and declining species. The experimental restoration of rare and endangered plant communities seems to be efficient and promising method for a future large-scale restoration projects in abandoned sand pits. -

Agathidium Vaderi MILLER & WHEELER, 2005
ZOBODAT - www.zobodat.at Zoologisch-Botanische Datenbank/Zoological-Botanical Database Digitale Literatur/Digital Literature Zeitschrift/Journal: Linzer biologische Beiträge Jahr/Year: 2021 Band/Volume: 0052_2 Autor(en)/Author(s): Schifko Georg Artikel/Article: Agathidium vaderi MILLER & WHEELER, 2005 (Coleoptera, Leiodidae) – Ein wissenschaftlicher Artname im Spannungsfeld der Zoologie, der Ethnologie und der Populärkultur 1099-1104 Linzer biol. Beitr. 52/2 1099-1104 Februar 2021 Agathidium vaderi MILLER & WHEELER, 2005 (Coleoptera, Leiodidae) – Ein wissenschaftlicher Artname im Spannungsfeld der Zoologie, der Ethnologie und der Populärkultur Georg SCHIFKO A b s t r a c t : In recent times, names related to popular culture have increasingly been given to new discovered species. This article deals with the Agathidium vaderi beetle named in 2005 by Kelly B. Miller and Quentin D. Wheeler after the fictional character Darth Vader due to the postulated similarity of the beetle's head with Darth Vader's helmet which is ultimately based on Japanese Samurai helmet designs. Additionally, attention is drawn to the the mutual benefits available to the taxonomy as well as for the films in the Star Wars series due to the unconventual species name. Key Words: Agathidium vaderi, Star Wars, Japanese helmets, Taxonomy, Nomenclature Einleitung Im Japanischen wird der zur Familie der Blatthornkäfer (Scarabaeidae) gehörende Allomyrina dichotoma (LINNAEUS, 1771) als kabutomushi bezeichnet, was soviel wie "Helminsekt" (kabuto = Helm, mushi = Insekt) bedeutet und eine Anspielung auf japanische Samuraihelme darstellt. A. dichotoma bildet im Land der aufgehenden Sonne einen fest verankerten Bestandteil der Populärkultur und man lässt dort Männchen dieser Spezies gegeneinander kämpfen. In den Wettbewerben geht es darum, wessen Käfer die schwerste Last ziehen kann und um Spiele, bei denen man die Käfermännchen dazu bringt um ein Stück Wassermelone zu kämpfen (LAURENT 2001: 70) – somit "eine Insekten- version des Sumo-Ringens" (HERZOG 2012: 57). -

The Biodiversity of Flying Coleoptera Associated With
THE BIODIVERSITY OF FLYING COLEOPTERA ASSOCIATED WITH INTEGRATED PEST MANAGEMENT OF THE DOUGLAS-FIR BEETLE (Dendroctonus pseudotsugae Hopkins) IN INTERIOR DOUGLAS-FIR (Pseudotsuga menziesii Franco). By Susanna Lynn Carson B. Sc., The University of Victoria, 1994 A THESIS SUBMITTED IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES (Department of Zoology) We accept this thesis as conforming To t(p^-feguired standard THE UNIVERSITY OF BRITISH COLUMBIA 2002 © Susanna Lynn Carson, 2002 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. 1 further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department The University of British Columbia Vancouver, Canada DE-6 (2/88) Abstract Increasing forest management resulting from bark beetle attack in British Columbia's forests has created a need to assess the impact of single species management on local insect biodiversity. In the Fort St James Forest District, in central British Columbia, Douglas-fir (Pseudotsuga menziesii Franco) (Fd) grows at the northern limit of its North American range. At the district level the species is rare (representing 1% of timber stands), and in the early 1990's growing populations of the Douglas-fir beetle (Dendroctonus pseudotsuage Hopkins) threatened the loss of all mature Douglas-fir habitat in the district. -

Durham E-Theses
Durham E-Theses Ecological Changes in the British Flora WALKER, KEVIN,JOHN How to cite: WALKER, KEVIN,JOHN (2009) Ecological Changes in the British Flora, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/121/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk Ecological Changes in the British Flora Kevin John Walker B.Sc., M.Sc. School of Biological and Biomedical Sciences University of Durham 2009 This thesis is submitted in candidature for the degree of Doctor of Philosophy Dedicated to Terry C. E. Wells (1935-2008) With thanks for the help and encouragement so generously given over the last ten years Plate 1 Pulsatilla vulgaris , Barnack Hills and Holes, Northamptonshire Photo: K.J. Walker Contents ii Contents List of tables vi List of figures viii List of plates x Declaration xi Abstract xii 1. -

Bees and Wasps of the East Sussex South Downs
A SURVEY OF THE BEES AND WASPS OF FIFTEEN CHALK GRASSLAND AND CHALK HEATH SITES WITHIN THE EAST SUSSEX SOUTH DOWNS Steven Falk, 2011 A SURVEY OF THE BEES AND WASPS OF FIFTEEN CHALK GRASSLAND AND CHALK HEATH SITES WITHIN THE EAST SUSSEX SOUTH DOWNS Steven Falk, 2011 Abstract For six years between 2003 and 2008, over 100 site visits were made to fifteen chalk grassland and chalk heath sites within the South Downs of Vice-county 14 (East Sussex). This produced a list of 227 bee and wasp species and revealed the comparative frequency of different species, the comparative richness of different sites and provided a basic insight into how many of the species interact with the South Downs at a site and landscape level. The study revealed that, in addition to the character of the semi-natural grasslands present, the bee and wasp fauna is also influenced by the more intensively-managed agricultural landscapes of the Downs, with many species taking advantage of blossoming hedge shrubs, flowery fallow fields, flowery arable field margins, flowering crops such as Rape, plus plants such as buttercups, thistles and dandelions within relatively improved pasture. Some very rare species were encountered, notably the bee Halictus eurygnathus Blüthgen which had not been seen in Britain since 1946. This was eventually recorded at seven sites and was associated with an abundance of Greater Knapweed. The very rare bees Anthophora retusa (Linnaeus) and Andrena niveata Friese were also observed foraging on several dates during their flight periods, providing a better insight into their ecology and conservation requirements.