EVIDENCE-BASED OUTCOMES CENTER Skin & Soft Tissue Infection (SSTI)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Approach to the Patient with Presumed Cellulitis Daniela Kroshinsky, MD,* Marc E
Approach to the Patient With Presumed Cellulitis Daniela Kroshinsky, MD,* Marc E. Grossman, MD, FACP,† and Lindy P. Fox, MD‡ Dermatologists frequently are consulted in the evaluation and management of the patient with cellulitic-appearing skin. For routine cellulitis, the clinical presentation and patient symptoms are usually sufficient for an accurate diagnosis. However, when the clinical presentation is somewhat atypical, or if the patient fails to respond to appropriate therapy for cellulitis because of routine bacterial pathogens, the differential diagnosis should be rapidly expanded. We discuss the approach to the patient with presumed cellulitis, with an emphasis on the differential diagnosis of cellulitis in both the immunocompetent and immunucompromised patient. Semin Cutan Med Surg 26:168-178 © 2007 Elsevier Inc. All rights reserved. KEYWORDS cellulitis, erysipelas 53-year-old woman with a history of recurrent breast of cellulitis, and telangiectasia and scattered enlarged mes- Acancer diagnosed 2 years before presentation and treated enchymal cells, characteristic of radiation changes. with radiation and chemotherapy (docetaxel, anastrozole, exemestane, gemcitabine) most recently 6 months before presentation was admitted for 3 weeks of worsening chest Clinical Problem wall pain and a rash over her mastectomy scar. Despite 5 days Dermatologists frequently are consulted in the evaluation of empiric antibiotic therapy with doxycycline and vancomy- and management of the patient with cellulitic-appearing cin, the chest wall erythema and pain were increasing. A dermatology consultation was called. An ulceration and sur- skin. Although the dermatologist may be consulted early on rounding erythematous papules were concentrated over the in the patient’s course, more often a dermatology consult is mastectomy scar with ill-defined erythematous patches that requested when a patient fails to respond to treatment. -

Bacterial Infections and Infectious Dermatologic Emergencies.Pdf
Learning Objectives Common Bacterial Infections recognition treatment complications Infectious Dermatologic Emergencies Necrotizing Fasciitis Toxic Shock Syndromes Normal Skin Flora Major function is to prevent skin infections Provides ecological competition for pathogens Hydrolyzes the lipids in sebum into free fatty acids which are toxic to many bacteria- linoleic and linolenic acid are more inhibitory of Staph Aureus Antimicrobial Peptides from lamellar bodies, Cathelicidins, and Defensins function to control overgrowth of pathogens Normal Skin Flora Aerobic Cocci Staphylococcus epidermidis Most common coccus on human skin All body sites, especially intertriginous areas Staphylococcus aureus More common in Atopic Dermatitis, Diabetes Mellitus, Hemodialysis, IVDU, Liver Disease, and HIV resident or contaminant? anterior nares- 20-35% perineum- 20% axillae and toe webs- 5-10% Normal Skin Flora Aerobic Coryneform Bacteria Corynebacterium minutissimum- intertriginous sites Erythrasma Anaerobic Coryneform Bacteria Propionibacterium acnes- sebaceous glands, hair follicles Acne vulgaris Gram Negative Bacteria Acinetobacter species- axillae, perineum, antecubital fossae - Requires moisture and maceration which increases pH and CO2 levels Yeast Pityrosporum ovale/Malassezia furfur- sebaceous sites Tinea Versicolor Introduction Strep and Staph cause the majority of skin infections in immunocompetent patients Immunodeficiency and underlying systemic disease result in severe infections which tend to be refractory to -

World Journal of Clinical Pediatrics
World Journal of W J C P Clinical Pediatrics Submit a Manuscript: http://www.f6publishing.com World J Clin Pediatr 2018 October 25; 7(4): 89-104 DOI: 10.5409/wjcp.v7.i4.89 ISSN 2219-2808 (online) REVIEW Perianal infectious dermatitis: An underdiagnosed, unremitting and stubborn condition Elena Daniela Serban Elena Daniela Serban, 2nd Department of Pediatrics, “Iuliu ficial inflammation of the perianal skin, which is of bac Hatieganu” University of Medicine and Pharmacy, Emergency terial origin (classically, group A betahemolytic strepto Hospital for Children, Cluj-Napoca 400177, Romania cocci). This narrative review aims to critically review and summarize the available scientific literature regarding ORCID number: Elena Daniela Serban (0000-0003-0906-1232). pediatric PID, being the first of its kind, to the best of Author contributions: Serban ED contributed to the paper’s the author’s knowledge. It also reports the first cases of conception and design, the data collection, extraction, analysis Romanian children with PID. Multiple databases were and evaluation, the interpretation of results, and the manuscript’s subjected to systematic literature search (from 1966 preparation, critical revision, editing and final submission. to April 30, 2018) to identify studies and case reports of children with PID. As such, this review provides up Conflict-of-interest statement: The author declares there are no dated information about essential aspects of PID (epi potential conflicts of interest relevant to this publication. demiology, etiology, pathogenesis, as well as clinical OpenAccess: This article is an open-access article which was features, required investigations and therapeutic options) selected by an in-house editor and fully peer-reviewed by external and of diagnostic pitfalls. -

Reading List 2012.Indd
General Reading iGAS Guidelines - Published January 2012 CLICK HERE Educational Interim UK guidelines for management of close community contacts of invasive group A streptococcal disease. Health Protection Agency, Workshops 2012 Group A Streptococcus Working Group. Communicable Disease and Public Health 2004; 7(4):354-361. CLICK HERE Keynote Presentation: Diagnosis and Complicated infections of skin and skin structures: when the infection is more than skin deep. DiNubile MJ, Lipsky, B. Journal of treatment Antimicrobial Chemotherapy, 2004, 53, Suppl. S2, ii37-ii50 of skin and soft CLICK HERE Practice guidelines for the diagnosis and management of skin and tissue infections soft tissue infections. Stevens DL et al. Clinical Infectious Disease 2005; 41:1373–1406 CLICK HERE Infections of skin and soft tissue: Outcomes of a classifi cation scheme. Eron J. Clinical Infectious Diseases 2000;31:287(A432). CLICK HERE Occurrence and antimicrobial susceptibility patterns of pathogens isolated from skin and soft tissue infections: report from the SENTRY READING Antimicrobial Surveillance Program (United States and Canada, 2000). Rennie RP et al. Diagn Microbiol Infect Dis. 2003 Apr; 45(4):287-293. LIST CLICK HERE Comparison of community and health care associated methicillin resistant Staphylococcus aureus infection. Naimi TS, et al. JAMA 2003; 290: 2976-2984 CLICK HERE Methicillin resistant S. aureus infections amoung patients in the emergency department. Moran GJ et al. The New England Journal of Medicine 2006 CLICK HERE HPR 2011;5(7): News CLICK HERE Polyclonal multiply antiobiotic-resistant methicillin-resistant Staphylococcus aureus with Panton-Valentine leucocidin in England. JAC 2009; doi: 10.1093/jac/dkp386; CLICK HERE Eff ect of antibiotics on Staphylococcus aureus producing panton- valentine leukocidin. -

Evidence-Based Management of Skin and Soft-Tissue Infections In
VISIT US AT BOOTH # 203 AT THE ACEP PEDIATRIC ASSEMBLY IN NEW YORK, NY, MARCH 24-25, 2015 February 2015 Evidence-Based Management Volume 12, Number 2 Authors Of Skin And Soft-Tissue Jennifer E. Sanders, MD Pediatric Emergency Medicine Fellow, Department of Emergency Medicine, Icahn School of Medicine at Mount Sinai, Infections In Pediatric Patients New York, NY Sylvia E. Garcia, MD Assistant Professor of Pediatrics and Pediatric Emergency In The Emergency Department Medicine, Icahn School of Medicine at Mount Sinai, New York, NY Abstract Peer Reviewers Jeffrey Bullard-Berent, MD, FAAP, FACEP Skin and soft-tissue infections are among the most common condi- Health Sciences Professor, Emergency Medicine and Pediatrics, University of California – San Francisco, Benioff tions seen in children in the emergency department. Emergency de- Children’s Hospital, San Francisco, CA partment visits for these infections more than doubled between 1993 Carla Laos, MD, FAAP and 2005, and they currently account for approximately 2% of all Pediatric Emergency Medicine Physician, Dell Children’s Hospital, Austin, TX emergency department visits in the United States. This rapid increase CME Objectives in patient visits can be attributed largely to the pervasiveness of community-acquired methicillin-resistant Staphylococcus aureus. The Upon completion of this article, you should be able to: 1. Describe the pathophysiology of community-acquired emergence of this disease entity has created a great deal of controver- methicillin-resistant Staphylococcus aureus. sy regarding treatment regimens for skin and soft-tissue infections. 2. Differentiate the clinical presentation of common skin and soft-tissue infections. This issue of Pediatric Emergency Medicine Practice will focus on the 3. -

56 AAVLD Diagnostic Pathology Slide Session
56th AAVLD Diagnostic Pathology Slide Session American Association of Veterinary Laboratory Diagnosticians San Diego, California Saturday, October 19, 2013 3:30-6:00 PM 56th AAVLD Diagnostic Pathology Slide Seminar 56thAAVLD Diagnostic Pathology Slide Session October 19, 2013 San Diego, California 2013 AAVLD Diagnostic Pathology Slide Seminar Presenters Case # Presenter Species Institution 1 Marcia R. S. Ilha Canine TVDIL-UGA 2 Alison Tucker Swine NCVDLS 3 Scott D. Fitzgerald Raccoon DCPAH-MSU 4 Bailey L. Wilberts Bovine ISU 5 Kelly Hughes Canine OSU-VDL 6 Dodd Sledge Chicken DCPAH-MSU 7 Tuddow Thaiwong Canine DCPAH-MSU 8 Kelli Almes Feline KSVDL-KSU 9 Chanran Ganta Swine KSVDL-KSU 10 Mahogany Caesar Equine NCVDLS 11 Panayiotis Loukopoulos Pacific gopher snake CAHFS-UCDavis 12 Andrew Brooks Canine AHL-UofG 13 Donal O’Toole Canine WSVL-UW 14 Sandra Scholes Bovine AHVLA 15 Leslie W. Woods Pacific fisher CAHFS-UCDavis 16 Francisco A. Uzal Bovine CAHFS-UCDavis 17 Jeffrey R. Hayes Swine Ohio ADDL 18 Tim Cushing Feline CVDC 2 56th AAVLD Diagnostic Pathology Slide Seminar 56thAAVLD Diagnostic Pathology Slide Session October 19, 2013 San Diego, California 2013 AAVLD Diagnostic Pathology Slide Seminar Diagnoses Case # Presenter Species Diagnosis Page # 1 Ilha Canine Extra-adrenal paraganglioma 4 2 Tucker Swine Idiopathic vesicular disease of swine 5 3 Fitzgerald Raccoon Canine distemper and infectious canine hepatitis 6 4 Wilberts Bovine Mannheimiosis and adenoviral infection 7 5 Hughes Canine Canine meningeal polyarteritis 8-9 6 Sledge Chicken -

Supplementary Information for 'Effect of Common Infections on the Incidence of Post-Stroke Dementia: a Cohort Study Using
Supplementary information for ‘Effect of common infections on the incidence of post-stroke dementia: a cohort study using the Clinical Practice Research Datalink’ Contents Appendix 1: Read code lists for major infections (lower respiratory tract infection, urinary tract infection, skin and soft tissue infection) ........................................................................... 2 LRTI codelist ........................................................................................................................... 2 UTI codelist ............................................................................................................................ 7 SSTI codelist ........................................................................................................................... 9 Appendix 2: Read code list for dementia................................................................................. 15 Appendix 3: Multivariable Cox regression models for early and late dementia by all characteristics included in analysis .......................................................................................... 19 Appendix 4: Multivariable Cox regression model for sensitivity analysis excluding infections in first 3 months ....................................................................................................................... 20 Appendix 5: Multivariable Cox regression model for effect of first infection type on dementia ................................................................................................................................. -

Subject Index to Abstracts
003 1-3998/85/1904-44 lA$02.00 Vol. 19, No. 4, 1985 PEDIATRIC RESEARCH Printed in U.S.A. Copyright O 1985 International Pediatric Research Foundation,Inc. SUBJECT INDEX TO ABSTRACTS 1,25 (OH)2 D 1200 ADENOSINE DEAMINASE 909, 962, ALCOHOLISM 514 1,25 DIHYDROXYVITAMIN D3 973 967 ALDOSTERONE 333, 334, 808, 1,251 DIHYDROXYVITAMIN D 1223, ADENOSINE TRIPHOSPHATASE 323, 1591, 1605 1681 1571 ALKALINE PHOSPHATASE 728, 1200 14C DEOXYGLUCOSE 1316 ADENOSINE TRIPHOSPHATE 90, 98, ALKALOSIS 796 17 HYDROXYPROGESTERONE 436 860, 1600 ALKALOSIS, RESPIRATORY 1409 21 HYDROXYLASE DEFICIENCY 861 ADENOTONSILLECTOMY 1725 ALLERGY 652 21 HYDROXYLASE DEFICIENCY, ADENOVIRUS INFECTIONS 950 ALLOGENEIC 1018 NONCLASSICAL 861 ADENOVIRUSES, HUMAN 585 ALLOGRAFT REJECTION 1647 24 HOUR 440 ADENYL CYCLASE 277, 439, 1592 ALLOGRAFTS 1593, 1593 24 HYDROXYLASE RESPONSE 1200 ADHERENCE 880 ALMITRINE 837 25 (OH) D 1200 ADHESION 309 ALOPECIA 973 25 HYDROXYVITAMIN D 1 HYDROXYLASE ADHESIVE GLYCOPROTEINS 880 ALPHA 1-ANTITRYPSIN 1822 1223 ADIPOCYTE 713 ALPHA 1-ANTITRYPSIN DEFICIENCY 2H (OH) D 1200 ADIPOSE TISSUE 1187 813 3 BETAHYDROXYSTEROID ADIPOSITY 713 ALPHA FETOPROTEINS 1296, 1305 DEHYDROGENASE 431 ADOLESCENT BEHAVIOR 10, 19 ALPHA MANNOSIDASE 1241 3 METHYLGLUTACONIC ACID 823 ADOLESCENT PREGNANCY 20 ALPHA TOCOPHEROL 1466 3 METHYLGLUTACONIC ACIDURIA 823 ADRENAL 275, 334 ALTERNATE DAY STEROIDS 1726 3 METHYLGLUTARIC ACID 823 ADRENAL ANDROGEN SECRETION 480 ALTITUDE 273 4 CHANNEL PNEUMOGRAM 1523 ADRENAL CORTICOSTEROIDS 454 ALUMINUM 1595 5' NUCLEOTIDASE 881 ADRENAL HYPERPLASIA 431, 467 ALUMINUM -

Mallory Prelims 27/1/05 1:16 Pm Page I
Mallory Prelims 27/1/05 1:16 pm Page i Illustrated Manual of Pediatric Dermatology Mallory Prelims 27/1/05 1:16 pm Page ii Mallory Prelims 27/1/05 1:16 pm Page iii Illustrated Manual of Pediatric Dermatology Diagnosis and Management Susan Bayliss Mallory MD Professor of Internal Medicine/Division of Dermatology and Department of Pediatrics Washington University School of Medicine Director, Pediatric Dermatology St. Louis Children’s Hospital St. Louis, Missouri, USA Alanna Bree MD St. Louis University Director, Pediatric Dermatology Cardinal Glennon Children’s Hospital St. Louis, Missouri, USA Peggy Chern MD Department of Internal Medicine/Division of Dermatology and Department of Pediatrics Washington University School of Medicine St. Louis, Missouri, USA Mallory Prelims 27/1/05 1:16 pm Page iv © 2005 Taylor & Francis, an imprint of the Taylor & Francis Group First published in the United Kingdom in 2005 by Taylor & Francis, an imprint of the Taylor & Francis Group, 2 Park Square, Milton Park Abingdon, Oxon OX14 4RN, UK Tel: +44 (0) 20 7017 6000 Fax: +44 (0) 20 7017 6699 Website: www.tandf.co.uk All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP. Although every effort has been made to ensure that all owners of copyright material have been acknowledged in this publication, we would be glad to acknowledge in subsequent reprints or editions any omissions brought to our attention. -
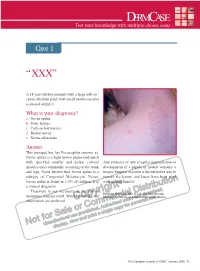
DERMCASE Test Your Knowledge with Multiple-Choice Cases
DERMCASE Test your knowledge with multiple-choice cases Case 1 “XXX” A 14 year-old boy presents with a large well-cir- cumscribed tan patch with small brown macules scattered within it. What is your diagnosis? a. Nevus spilus b. Solar lentigo c. Café-au-lait macule d. Becker nevus e. Nevus sebaceous Answer This teenaged boy has Nevus spilus (answer a). Nevus spilus is a light brown pigmented patch with speckled smaller and darker colored Any evidence of new irregular pigmentation or macules most commonly occurring on the trunk development of a papule or nodule warrants a and legs. Some believe that Nevus spilus is a biopsy. Surgical excision is the definitive way to subtype of Congenital Melanocytic Nevus. remove the lesion, and lasers have been tried Nevus spilus is found in 1-2% of children. It is with modest benefit. a clinical diagnosis. Treatment is not necessary as the risk of Benjamin Barankin, MD, is a Senior Dermatology melanoma remains small. Watchful waiting and Resident, University of Alberta, Edmonton, Alberta. observation are preferred. The Canadian Journal of CME / January 2006 71 DERMCASE Case 2 What’s on my head? This infant presents with a greasy, scaly lesion on the forehead and scalp. There is no history of diarrhea or failure to thrive. The infant does not appear to be in discomfort. What is it?? a. Atopic dermatitis b. Infantile seborrheic dermatitis c. Psoriasis d. Leiner’s disease Answer Infantile seborrheic dermatitis (answer b) usually manifests with erythema on the scalp, forehead, and retro-auricular areas. The erythema is typically covered with greasy, Infantile seborrheic dermatitis usually yellow scale. -
Current Essentials : Pediatrics
a LANGE medical book CURRENT ESSENTIALS PEDIATRICS Judith M. Sondheimer, MD Professor Emeritus Department of Pediatrics Section of Pediatric Gastroenterology, Hepatology and Nutrition University of Colorado School of Medicine The Children’s Hospital Denver, Colorado New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto Copyright © 2008 by The McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. 0-07-151082-6 The material in this eBook also appears in the print version of this title: 0-07-141256-5. All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at [email protected] or (212) 904-4069. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. (“McGraw-Hill”) and its licensors reserve all rights in and to the work. -

A Study to Compare the Possible Antifungal and Antimicrobial Effects
COPYRIGHT AND CITATION CONSIDERATIONS FOR THIS THESIS/ DISSERTATION o Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use. o NonCommercial — You may not use the material for commercial purposes. o ShareAlike — If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original. How to cite this thesis Surname, Initial(s). (2012) Title of the thesis or dissertation. PhD. (Chemistry)/ M.Sc. (Physics)/ M.A. (Philosophy)/M.Com. (Finance) etc. [Unpublished]: University of Johannesburg. Retrieved from: https://ujdigispace.uj.ac.za (Accessed: Date). A STUDY TO COMPARE THE POSSIBLE ANTIFUNGAL AND ANTIMICROBIAL EFFECTS OF A HOMOEOPATHIC COMPLEX OF ECHINACEA ANGUSTIFOLIA • ECHINACEA PURPUREA WITH THAT OF SINGLE ENTITY REMEDIES PREPARED FROM ECHINACEA ANGUSTIFOLIA AND ECHINACEA PURPUREA, RESPECTIVELY by KANELLIE HATZIKONSTANDINOU Student No. 8921415 \ ,A Dissertation submitted to the Faculty of Health Sciences, Technikon Witwatersrand in partial fulfilment of the requirements for the degree of Masterin Technology: Homoeopathy Johannesburg, 2000 DECLARATION I, KANELLIE HATZIKONSTANDINOU, declare that this Dissertation is my own work. It is being submitted for the degree of Master in Technology: Homoeopathy at the Technikon Witwatersrand, Johannesburg. It has not been submitted before for any degree or examination at this or any other Tertiary institution. Signe~ this .4....... day of ":;;'''''1'' ..... 2000. f '-.. .... _- "'--'-~"~- _.._-----_._-- ............... ------.. - ii In loving memory of my Mother, Thelma and with love and appreciation to my beloved Father, loannis, without who's continued support this journey would not have been so fulfilling.