Draft Standards for Sugar Cane Wine (Basi)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Traditional Dietary Culture of Southeast Asia
Traditional Dietary Culture of Southeast Asia Foodways can reveal the strongest and deepest traces of human history and culture, and this pioneering volume is a detailed study of the development of the traditional dietary culture of Southeast Asia from Laos and Vietnam to the Philippines and New Guinea from earliest times to the present. Being blessed with abundant natural resources, dietary culture in Southeast Asia flourished during the pre- European period on the basis of close relationships between the cultural spheres of India and China, only to undergo significant change during the rise of Islam and the age of European colonialism. What we think of as the Southeast Asian cuisine today is the result of the complex interplay of many factors over centuries. The work is supported by full geological, archaeological, biological and chemical data, and is based largely upon Southeast Asian sources which have not been available up until now. This is essential reading for anyone interested in culinary history, the anthropology of food, and in the complex history of Southeast Asia. Professor Akira Matsuyama graduated from the University of Tokyo. He later obtained a doctorate in Agriculture from that university, later becoming Director of Radiobiology at the Institute of Physical and Chemical research. After working in Indonesia he returned to Tokyo's University of Agriculture as Visiting Professor. He is currently Honorary Scientist at the Institute of Physical and Chemical Research, Tokyo. This page intentionally left blank Traditional Dietary Culture of Southeast Asia Its Formation and Pedigree Akira Matsuyama Translated by Atsunobu Tomomatsu Routledge RTaylor & Francis Group LONDON AND NEW YORK First published by Kegan Paul in 2003 This edition first published in 2009 by Routledge 2 Park Square, Milton Park, Abingdon, Oxon, OX14 4RN Simultaneously published in the USA and Canada by Routledge 270 Madison Avenue, New York, NY 10016 Routledge is an imprint o f the Taylor & Francis Group, an informa business © 2003 Kegan Paul All rights reserved. -

A Cta ΠCumenica
2020 N. 2 ACTA 2020 ŒCUMENICA INFORMATION SERVICE OF THE PONTIFICAL COUNCIL FOR PROMOTING CHRISTIAN UNITY e origin of the Pontical Council for Promoting Christian Unity is closely linked with the Second Vatican Council. On 5 June 1960, Saint Pope John XXIII established a ‘Secretariat for Promoting Christian Unity’ as one of the preparatory commissions for the Council. In 1966, Saint Pope Paul VI conrmed the Secretariat as a permanent dicastery CUMENICA of the Holy See. In 1974, a Commission for Religious Relations with the Jews was established within the Secretariat. In 1988, Saint Pope John Paul II changed the Secretariats status to Pontical Council. Œ e Pontical Council is entrusted with promoting an authentic ecumenical spirit in the Catholic Church based on the principles of Unitatis redintegratio and the guidelines of its Ecumenical Directory rst published in 1967, and later reissued in 1993. e Pontical Council also promotes Christian unity by strengthening relationships CTA with other Churches and Ecclesial Communities, particularly through A theological dialogue. e Pontical Council appoints Catholic observers to various ecumenical gatherings and in turn invites observers or ‘fraternal delegates’ of other Churches or Ecclesial Communities to major events of the Catholic Church. Front cover Detail of the icon of the two holy Apostles and brothers Peter and Andrew, symbolizing the Churches of the East and of the West and the “brotherhood rediscovered” (UUS 51) N. 2 among Christians on their way towards unity. (Original at the Pontical -

A Substance Inhibiting the Growth of Lactic Acid Bacteria in Duhat (Sizygium Cumini Skeels) Bark
Biocontrol Science, 2000, Vol.5, No.1, 33-38 Original A Substance Inhibiting the Growth of Lactic Acid Bacteria in Duhat (Sizygium cumini Skeels) Bark KIYOSHI MURA*, HIDEAKI SHIRAMATSU, AND WAHACHIRO TANIMURA Faculty of Applied Bioscience, Tokyo University of Agriculture , 1-1-1 Sakuragaoka, Setagaya-ku, Tokyo 156-8502, Japan Received 2 April 1999/Accepted 8 September 1999 We isolated a substance inhibiting the growth of lactic acid bacteria from duhat bark, which is added to "basi" , a sugar cane wine in the Philippines, and investigated its structure . By adding gelatin to the aqueous extract of duhat bark (AEDB), 63.2% of the polyphenol compo- nents in AEDB were precipitated, and the resulting supernatant lost its inhibitory activity on the growth of lactic acid bacteria. This result indicates that the inhibitory substance was the polyphenol component combining to protein. In addition, we fractionated AEDB into two frac- tions by ultrafiltration and investigated their inhibitory activities on the growth of lactic acid bacteria. A strong inhibitory activity was found in the fraction having molecular weight (MW) above 1•~104 containing about 53% of the polyphenol components in AEDB, indicating that the main inhibitory substance is a polyphenol component with high MW. We then separated the polyphenol components with MW above 1•~104 by ion-exclusion chromatography using CM- Sepharose CL-6B, and obtained a polyphenol component with inhibitory activity on the growth of lactic acid bacteria. The polyphenol component produced gallic acid, anthocyanidins of delphinidin and cyanidin, and glucose by hydrolysis with HCl, and was assumed to be con- densed tannin comprised of gallic acid and leucoanthocyanin. -

Physicochemical Properties of Macaranga (Gamu) Used in Kalinga Basi (Bayas) Production Divina Alunday – Balocnit
International Journal of English Literature and Social Sciences, 5(6) Nov-Dec 2020 | Available online: https://ijels.com/ Physicochemical Properties of Macaranga (Gamu) used in Kalinga Basi (Bayas) Production Divina Alunday – Balocnit Kalinga State University, Philippines Received: 12 Oct 2020; Received in revised form: 24 Dec 2020; Accepted: 29 Dec 2020; Available online: 31 Dec 2020 ©2020 The Author(s). Published by Infogain Publication. This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/). Abstract— This study focused on assessing the physisco-chemical properties of Gamu used in Kalinga basi/ bayas production. Specifically, this study covers the ethnobotanical profile of the binuwa tree. The fruit of the binuwa tree is the gamu used as yeast to ferment in the production of bayas. Some of the chemical components of this fruit are also analyzed in this study. The physical and chemical characteristic of the bayas was also studied. This study's ultimate goal was to assess the physicochemical properties of gamu fruit used in basi/bayas production. Phytochemical analysis was used to get data on the chemical properties of the gamu and bayas. Survey methods and photo-documentation were applied to gather data on the physical characteristics of the local wines. The study found out that the binuwa tree is naturally grown in thick forests in the province and that its dried fruit, which is the gamu, is used as a starter to ferment basi/bayas. This fruit contains flavonoids and tannins responsible for antimicrobial activity and astringency of the wine, respectively. The basi/bayas have an alcohol content of 9.79%v/v, Reducing Sugars 4.7%w/w, pH of 3 – 4, and a total acid of 0.64g/100g of bayas. -

2006 Fishing Guide Layout
Estado de Illinois Departamento de Recursos Naturales de Illinois Carl Zitsman / USFWS Robert H. Pos / USFWS Robert H. Pos / USFWS DEPARTAMENTO DE RECURSOS NATURALES DE ILLINOIS Jo Daviess Stephenson Winnebago Boone Mc Henry Lake Carroll Ogle De Kalb Kane Cook Región ii Du Page Whiteside Lee 2050 West Stearns Rd. Kendall Will Bartlett, IL 60103 La Salle Henry Bureau REGION REGION (847) 608-3100 Rock Island Grundy Región i Mercer II Stark I Putnam Kankakee Knox 2317 E. Lincolnway Warren Livingston Marshall Peoria Iroquois Sterling, IL 61081 Woodford Henderson McLean (815) 625-2968 Fulton Tazewell Hancock Mc Donough Ford Vermilion Mason Champaign Región iii Logan Schuyler De Witt Adams Piatt REGION 1556 State Route 54 Menard Brown Cass Macon III Clinton, IL 61727-9360 Sangamon Morgan Douglas Edgar (217) 935-6860 Pike Scott Moultrie Christian Coles Shelby Greene Macoupin Calhoun Clark REGION Montgomery Cumberland Jersey Fayette Effingham IV Jasper Crawford Madison Bond Región iV Clay Richland Lawrence Marion 4521 Alton Commerce Pkwy. Clinton St. Clair Wayne Alton, IL 62002 Edwards Wabash Washington Jefferson REGION Región V (618) 462-1181 Monroe White 11731 Hgwy. 37 Randolph Perry V Franklin Hamilton Benton, IL 62812 Jackson Saline Gallatin Williamson (618) 435-8138 Union Johnson Pope Hardin Alexander Pulaski Massac PARA RECIBIR INFORMACIÓN ADICIONAL, CONTACTE A: Oficinas en Springfield Oficina en Chicago T.i.P. Departamento de Departamento de Target illinois Poachers Recursos naturales Recursos naturales Reporte las violaciones a: One Natural Resources Way James R. Thompson Center 1-877-2DNRLAW Springfield, IL 62702-1271 Suite 4-300 (236-7529) 100 West Randolph St. -

Production of Popular Native Wines in the Philippines
Production of Popular Native Wines in the Philippines by nobert soloria bermosa on Apr 25, 2008 Native wines produced in the Philippines are all 100% chemical-free because they are all produce in a natural process. If one day you happen to pay visit in the Philippines, this article will be of help to you especially if you are a native liquor or wine drinker or collector. Usually if we visit a certain place, we tend to get familiarize first with the kind of foods offered to us. Aside from the foods, the next thing that we get familiar with is the drinks. Food and drinks always go hand in hand. The following are list of wines locally manufactured in the Philippines. These are 100% chemical- free because they are all produce in natural methods. “Tapoy” or Rice Wine of Mountain Provinces Tapoy is similar to the rice wine of Japan called sake. This kind of wine is widely produce in the provinces of Cordilleras, specifically by the Ifugaos, the builder of the world -famous Rice Terraces, by the Igorots, and other ethnic tribes in the Mountain Provinces. This kind of wine is basically made by soaking raw glutinous rice in hot water for I hour. Drain and steam for 25 minutes and then spread in a tray to let it cool for 2 hours. The yeast and rice is then combined by hand until blended (as you can see in the picture). The mixture is then transferred in a container covered tightly with a lid and stored in a dry place and allow to ferment for 1 month. -

HILIGAYNON LESSONS PALI Language Texts: Philippines (Pacific and Asian Linguistics Institute) Howard P
HILIGAYNON LESSONS PALI Language Texts: Philippines (Pacific and Asian Linguistics Institute) Howard P. McKaughan Editor HILIGAYNON LESSONS by Cecile L. Motus University of Hawaii Press Honolulu 1971 Open Access edition funded by the National Endowment for the Humanities / Andrew W. Mellon Foundation Humanities Open Book Program. Licensed under the terms of Creative Commons Attribution-NonCommercial-ShareAlike 4.0 Inter- national (CC BY-NC-SA 4.0), which permits readers to freely download and share the work in print or electronic format for non-commercial purposes, so long as credit is given to the author. The license also permits readers to create and share de- rivatives of the work, so long as such derivatives are shared under the same terms of this license. Commercial uses require permission from the publisher. For details, see https://creativecommons.org/licenses/by-nc-sa/4.0/. The Cre- ative Commons license described above does not apply to any material that is separately copyrighted. Open Access ISBNs: 9780824882013 (PDF) 9780824882020 (EPUB) This version created: 20 May, 2019 Please visit www.hawaiiopen.org for more Open Access works from University of Hawai‘i Press. The work reported herein was performed pursuant to a contract with the Peace Corps, Washington, D.C. 20525. The opinions ex- pressed herein are those of the author and should not be con- strued as representing the opinions or policies of any agency of the United States government. Copyright © 1971 by University of Hawaii Press All rights reserved PREFACE These Lessons in Hiligaynon have been developed under the auspices of the Pacific and Asian Linguistics Institute of the Uni- versity of Hawaii with the help of a Peace Corps contract (PC 25–1507). -

Philippine Fermented Foods
PHILIPPINE FERMENTED F 0 0 D s PRINCIPLES AND TECHNOLOGY Philippine Fe rmented Foods Philippine Fermented Foods Principles an d Technology Priscilla Chinte-Sanchez, PhD The University of the Philippines Press Diliman, Quezon City THE UNIVERSITY OF THE PHILIPPINES PRESS E. de los Santos St., UP Campus, Diliman, Quezon City 1101 Te l. Nos.: 9282558, 9253243, 9266642 E-mail: [email protected] ©'2008 by Priscilla Chinte-Sanchez All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any fo rm or by any means, electronic,mechanical, photocopying, and/or otherwise, without the prior written permission of the author and the publisher. The NationalLibrary of the Philippines CIP Data Recommended entry: Sanchez, Priscilla Chinte. Philippine fermented fo ods: principles and technology/ Priscilla Chinte-Sanchez. Quezon City: The University of the Philippines Press, c2008. p.; em. 1. Fermented foods-Philippines-Handbooks, manuals, etc. 2. Fermentation-Handbooks, manuals, etc. I. Title. TP371.44 664.024 2008 P083000116 ISBN 978-971 -542-554-4 Book Design: Nicole Victoria Printed in the Philippines Dedicated to my husband, Fernando ("Nanding''), and children, FernandoJr. ("Dindo'') and Maria Matilde ("Nene''), as well as to Ronald and Patricia Ann, and, most of all, to my loving grandson, Tristan Fernando, for their love, encouragement, moral support, and understanding. vii Tab I e 0 f Co ntents List of Figures XVI List of Tables XVlll Preface XXl Section 1. Principles in Food Fermentation Chapter I. Food Preservation byFe rmentation Definitionand Importance of Fermentation Role of Fermented Foods in the Food Supply 2 Nutritional Significance of Fermented Foods 5 Ty pes of Fermentation 8 Diversity of Microbes in Fermented Foods 9 Chapter II. -

An Introduction
Tribute, Trade, and Smuggling: An Introduction Angela Schottenhammer On November 26 2011 we organized an international workshop at Ghent University, Belgium, entitled “Tribute, Trade, and Smuggling: Commercial, Scientific, and Human Interaction in the Middle Period and Early Modern World”. Our aim was to focus particularly on wide and active networks of unofficial, private or illegal commercial exchange activities and knowledge transfer, including the trafficking of people as well as human interaction that took place behind the offi- cial curtain of tribute and trade. Simultaneously, we intended to compare examples from Asia with those from other parts of the world. So, when the idea to produce this book began, it was clear that we would not simply publish a workshop proceeding but to select papers and ask other scholars to contribute to this volume to give it its final shape. Officially, relations between a so-called empire like China and her neighbouring countries not infrequently were designated as “tribute relations”. Much of the commercial and scientific intercourse that was going on not only in Asia but in the middle period and early modern world in general, however, followed illegal or “private” paths and channels. Commodities, products, knowledge or human beings entered or left a country without the explicit permission or ap- proval of a government or an official institution. Accordingly, this volume is divided into the sections commercial, scientific and human interaction. The emphasis of this volume definitely lies on East Asia and almost each contribution at least relates to this macro-region. But we hope that the few examples from other parts of the world may provide a comparative perspective showing that these clandestine networks were active world-wide and heavily influenced international and supra-regional exchange relations and interaction. -

AHMED AMÎŞ EFENDİ Kaddese’Llâhü Sırrahu’L Azîz
FATİH SERTÜRBEDÂRI TIRNOVALI Kutbu’l-Ârifîn, Gavsu’l-Vâsilîn MÜRŞİD-İ KÂMİL AHMED AMÎŞ EFENDİ Kaddese’llâhü sırrahu’l azîz TAKDİM Halvetiyye-i Şa'bâniyye yolunun mürşid-i Kâmille- rinden, 1920 yılında sırlanan Fatih sertürbedârı Ahmed Amîş Hazretlerinin sohbet ve nasihatlerinin güneş ışığı gibi tüm gönüllerde nasîbdar olmasını Cenab-ı Allah'tan niyaz ediyorum. H. S. Ç. 2011 Kasım - Kurban Bayramı "Taş taş olmuş yere yatmış, onun kaderinde basıl- mak var. Ama sen, sen ol yolda bir taş gördüğün zaman, sakın onu ayağınla itme! Elinle bir kenara bırak" Derleyen: İsmail Hakkı Altuntaş Kapak: Bülent Engez Baskı: Seçil Ofset - İstanbul Matbaamız tarafından basılan bu değerli eseri, ücretsiz olarak temin edebilirsiniz TEL: 0212 629 06 15 www. secilofset. com info @ secilofset. com İçindekiler KUTBU'L-VÂSILÎN AHMED AMÎŞ EFENDİ ................ 9 Abdulhamid Han .............................................. 19 Adab ................................................................. 20 Adak ................................................................. 23 Ahlak ................................................................ 23 Allah Teâlâ; ....................................................... 23 Ana-Baba hakkı ................................................ 24 Besmele ............................................................ 24 Biat ................................................................... 24 Borç .................................................................. 25 Buğz ................................................................. -

Parshas Tazria, Parshas Hachodesh, Pesach
בס‘‘ד the gift that Na’aman offered, but Geichazi accepted it. As punishment, he and his sons were cursed by Elisha to be plagued with the Tzara’as of Na’aman, and therefore they had to live outside the city (of Shomron, the capital city of the Ten Tribes). This turned out to be the Refuah Kodem L’makah, because later when Shomron was be- sieged, these men were in the right place to discover the treasure of food in the aban- doned camp of Aram just in time to save the city’s inhabitants from death by starva- Volume 11: Issue 22 1 Nissan, 57756 tion. Parshas Tazria, Parshas HaChodesh, Pesach April 9, 2016 The miracle is compounded when we learn a fascinating vort quoted in K’motzei Shalal Rav and realize that they didn’t even have to be there Halachically. The Mishna Parshas Tazria, Parshas HaChodesh, Pesach (Keilim 1:7) says that a Metzora (leper) must be evicted only from a city that is sur- rounded by a wall, and the Bartenura says that means a wall that was in existence since the time of Yehoshua Bin Nun. R’ Akiva Eiger asks, Shomron didn’t even exist in Yehoshua’s time; it was built by Omri, the grandfather of the then-current king Ye- horam, to be the capital of the Ten Tribes. Binyan Tzion answers that in truth there was no obligation to send a Metzora out of that city; they did so anyway because they wanted their capital city to be just like Yerushalayim. -
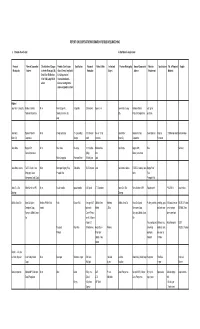
Report on Cooperative Information for Business Matching
REPORT ON COOPERATIVE INFORMATION FOR BUSINESS MATCHING a. Products Classification b. Raw Materails requirements Province/ Name of Cooperative / Classification of Coop as Products Classification Specification Volume of Market Outlets Institutional Province /Municipality Name of Cooperative/ Materials Specifications Vol. of Required Supplier Municipality Address to Assets Micro up to 3M, Grains/ Cereals/ veg/ fruits/ Production Buyers Address Requirement Materials Small 3 to 15M, Medium fish & Aquamarine/ 15 to 100M, Large 100 M livestock/ furniture & above fixtures/ noveltyy items/ processed products, others Region I Iloco Norte / Laoag City Kailokuan Saniata Micro Fresh Dragon Fruit 3-5 pgs/kilo 200 tons/year Supermarket Ilocos Norte / Laoag Kailokuan Saniata cuttings for Producers Cooperative Cookies, ice cream, etc. City Producers Cooperative plantation soap Iloco Norte/ Bapamin Farmer's Micro Vinegar and syrup 5% (Low acidity) 1,000 liters per Souvenir Shop Ilocos Norte / Bapamin Farmers Sweet Sorghum Naturally 1,000/liters per month Members/coop Batac City Cooperative Vinegar month Groceries Batac City Cooperative Fermented Ilocos Norte Bagnos MPC Micro Rice Coffee Beverage 11,500 bottles Robinson Bus Ilocos Norte Bagnos MPC Rice Members Banna, Ilocos Norte (300g) Gold Banna, Ilocos Norte Native Longganisa Processed Meat 600 kilos/year Local Ilocos Norte / Adams TadECC Credit Coop Micro Beverages: Bugnay, Rice, Table Wine 30,000 ltrs/year Local Ilocos Norte / Adams TADECCO, Adams, Ilocos Bugnay Fruit/ Itimpuyog ti Adams Pineaplle