Juglans Regia L.) Genetic Resources in Montenegro
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
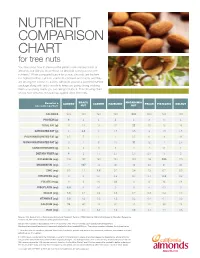
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

AP-42, CH 9.10.2.2: Peanut Processing
9.10.2.2 Peanut Processing 9.10.2.2.1 General Peanuts (Arachis hypogaea), also known as groundnuts or goobers, are an annual leguminous herb native to South America. The peanut peduncle, or peg (the stalk that holds the flower), elongates after flower fertilization and bends down into the ground, where the peanut seed matures. Peanuts have a growing period of approximately 5 months. Seeding typically occurs mid-April to mid-May, and harvesting during August in the United States. Light, sandy loam soils are preferred for peanut production. Moderate rainfall of between 51 and 102 centimeters (cm) (20 and 40 inches [in.]) annually is also necessary. The leading peanut producing states are Georgia, Alabama, North Carolina, Texas, Virginia, Florida, and Oklahoma. 9.10.2.2.2 Process Description The initial step in processing is harvesting, which typically begins with the mowing of mature peanut plants. Then the peanut plants are inverted by specialized machines, peanut inverters, that dig, shake, and place the peanut plants, with the peanut pods on top, into windrows for field curing. After open-air drying, mature peanuts are picked up from the windrow with combines that separate the peanut pods from the plant using various thrashing operations. The peanut plants are deposited back onto the fields and the pods are accumulated in hoppers. Some combines dig and separate the vines and stems from the peanut pods in 1 step, and peanuts harvested by this method are cured in storage. Some small producers still use traditional harvesting methods, plowing the plants from the ground and manually stacking them for field curing. -

Pistachio and Walnut Development Project TCP/KYR/3203 (D)
State Agency of Forestry and Environmental Protection, Kyrgyz Republic Pistachio and Walnut Development Project TCP/KYR/3203 (D) Hafiz Muminjanov Plant Production & Protection Officer Food and Agriculture Organization of the United Nations Sub-regional Office for Central Asia (FAOSEC) Agriculture in Kyrgyz Republic • Cereals - 1.5 mln MT, • including wheat 0.7 mln. MT • Potato - 1. 3 mln. MT • Vegetables - 0.8 mln. MT • Fruits -200 000 MT • Grapes - 10 000 MT 1 Why pistachio and walnut? Total Plantation Production, Yield, Area, ha Area, ha MT kg/ha Pistachio 57 000 12000 1 000 15-20 Walnut 47 000 - 900-1 900 20-40 Agriculture growth vs Overall Growth Kyrgyz Republic - Ag. Growth Vs. Overall Growth 160 Total GDP (1990=100) 140 AgGDP (1990=100) Total GDP (2002=100) AgGDP (2002=100) 120 100 AgGDP 80 (1990=100) Total GDP 60 (1990=100) 40 2 Wheat Grain Trade Balance ('000 tons) 1,200 1,000 800 600 Import 400 Domestic Production 200 - 2003 2004 2005 2006 2007 Trade Balance Values Dry Nuts Vs. Agriculture (2001 = 100) 600 Dry Nuts 400 200 0 2001 2002 2003 2004 2005 2006 - 200 - 400 Agriculture - 600 3 Why pistachio and walnut? • Central Asia – centre of origin & diversity of nuts • Large area of forest plantations • Suitable climatic conditions (dry summer) • Suitable socio-economic environment • Organic forests 4 Why pistachio and walnut? • Absence of market orientation in the past • Current varieties not valued for international market • No management done (cutting, pruning, etc.) • Density is high, pistachio male/female ratio not regulated • Yield is low • History of quality produce • International standards (USA, France, Chile, Australia) • Demand increase • Potential for export • Increasing farmers income & Livelihood improvement 5 Why pistachio and walnut? Development of the nut fruit sector has a high potential in improving livelihood and food security due the high international demand… Past and Related Work • Tien Shan Ecosystem Development Project (IFAD; GEF; WB-Bio Carbon Fund; Japanese Government). -
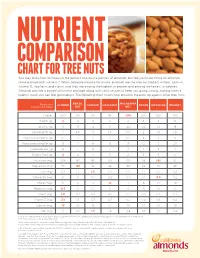
Chart for Tree Nuts
NUTRIENT COMPARISON CHART FOR TREE NUTS You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the highest in protein and among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a healthy snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT Calories 1602 190 160 180 200 200 160 190 Protein (g) 6 4 4 4 2 3 6 4 Total Fat (g) 14 19 13 17 22 20 13 18 Saturated Fat (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 Polyunsaturated Fat (g) 3.5 7 2 2 0.5 6 4 13 Monounsaturated Fat (g) 9 7 8 13 17 12 7 2.5 Carbohydrates (g) 6 3 9 5 4 4 8 4 Dietary Fiber (g) 4 2 1 3 2 3 3 2 Potassium (mg) 208 187 160 193 103 116 285 125 Magnesium (mg) 77 107 74 46 33 34 31 45 Zinc (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 Vitamin B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 Folate (mcg) 12 6 20 32 3 6 14 28 Riboflavin (mg) 0.3 0 0.1 0 0 0 0.1 0 Niacin (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 Vitamin E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.6 0.2 Calcium (mg) 76 45 13 32 20 20 30 28 Iron (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Canarium Ovatum) Shells
International Journal of Engineering Research And Management (IJERM) ISSN: 2349- 2058, Volume-04, Issue-09, September 2017 Physical and Mechanical Properties of Particleboard Utilizing Pili Nut (Canarium Ovatum) Shells Ariel B. Morales are widely used for various purposes. It is a lightweight and Abstract— This study aims to make a 200 mm x 200 mm strong kind of plastic that can be easily remolded into particleboard with nine millimeter thickness using Crushed Pili different shapes [5]. This study also helps in lessening Nut Shells (CPNS) and sawdust as raw materials with the ratios non-biodegradable wastes and be useful as a resin. This study (CPNS: sawdust) 100:0, 75:25, and 50:50 by weight and determine their physical and mechanical properties. Also, the specifically aims the following objectives: results were compared to the standard properties of commercial • To determine its physical property such as density, unit particleboard set classified by the Philippine Standard weight and thickness swelling, Association (PHILSA). The raw materials were mixed with • To determine the mechanical property, specifically High-Density Polyethylene (HDPE) as an adhesive. All Modulus of Rupture (MOR). particleboards have passed and exceeded the PHILSA standard • To compare the results to the standard properties for for particle board where 100:0 mixtures with density of 1204.09 kg/m3 has the highest MOR of 110 MPa and lowest thickness commercial particleboard from Philippine Standard swelling of 1.11%. Therefore, particleboard made from Pili nut Association (PHILSA) if it will pass or exceed the given shells has an outstanding property compared to commercial standards. particleboards that depends on its application. -

Trailer Decontamination)
Preventing Peanut Cross-Contamination in Georgia Pecans (Trailer Decontamination) Peanut cross contamination within the pecan industry is a serious concern. One potential source of cross-contamination within the Georgia pecan industry is the use of trailers previously used in peanut harvest for transport of pecans during pecan harvest. Sixty three percent of all food-allergen related deaths in the U.S. are the result of peanut allergies. These allergies result from allergenic proteins found in the peanut kernel. If pecan growers were to use trailers which previously held peanuts during pecan harvest, the potential would exist for the pecans to be contaminated with peanut allergens through peanut oil residue left on the surface of the trailer or through left-over peanuts still remaining in the trailer from peanut harvest. If contaminated pecans were to be processed in a shelling plant, the entire shelling plant itself could become contaminated. Ultimately, a peanut allergin-related death traced back to the consumption of peanut contaminated pecans could have a devastating effect upon the Georgia pecan industry. Therefore, before pecans are dumped into trailers at harvest, the following steps are suggested for decontaminating trailers that may have previously been used in peanut harvest: 1. Clean & sweep trailer thoroughly, assuring that all previous products are removed from trailer. 2. Wash trailer with soap & water solution, making sure all surfaces are scrubbed well with a brush. Use any FDA approved food grade detergents. 3. Rinse trailer out thoroughly with water making sure there are no visual signs of cleaning solution left inside. 4. Sanitize inside of trailer with at least 50 ppm chlorine water solution. -

Comparative Proteomic Analysis of Walnut (Juglans Regia L.) Pellicle Tissues Reveals the Regulation of Nut Quality Attributes
life Article Comparative Proteomic Analysis of Walnut (Juglans regia L.) Pellicle Tissues Reveals the Regulation of Nut Quality Attributes Paulo A. Zaini 1, Noah G. Feinberg 1, Filipa S. Grilo 2 , Houston J. Saxe 1 , Michelle R. Salemi 3, Brett S. Phinney 3 , Carlos H. Crisosto 1 and Abhaya M. Dandekar 1,* 1 Department of Plant Sciences, University of California, Davis, CA 95616, USA; [email protected] (P.A.Z.); [email protected] (N.G.F.); [email protected] (H.J.S.); [email protected] (C.H.C.) 2 Department of Food Sciences and Technology, University of California, Davis, CA 95616, USA; [email protected] 3 Proteomics Core Facility, University of California, Davis, CA 95616, USA; [email protected] (M.R.S.); [email protected] (B.S.P.) * Correspondence: [email protected] Received: 2 November 2020; Accepted: 25 November 2020; Published: 27 November 2020 Abstract: Walnuts (Juglans regia L.) are a valuable dietary source of polyphenols and lipids, with increasing worldwide consumption. California is a major producer, with ‘Chandler’ and ‘Tulare’ among the cultivars more widely grown. ‘Chandler’ produces kernels with extra light color at a higher frequency than other cultivars, gaining preference by growers and consumers. Here we performed a deep comparative proteome analysis of kernel pellicle tissue from these two valued genotypes at three harvest maturities, detecting a total of 4937 J. regia proteins. Late and early maturity stages were compared for each cultivar, revealing many developmental responses common or specific for each cultivar. Top protein biomarkers for each developmental stage were also selected based on larger fold-change differences and lower variance among replicates, including proteins for biosynthesis of lipids and phenols, defense-related proteins and desiccation stress-related proteins. -

Tree Nut Allergen Component Testing Brochure
Walnut, cashew, and Brazil nut allergen component testing Jug r1 Walnut, Jug cashew, and r3 Brazil nut allergens by Ana o3 the numbers Ber e1 Detect sensitizations to the whole nut to create personalized management plans for your patients. Allergen component testing Measurement of specific IgE by blood test that provides objective assessment of sensitization to the whole nut protein is the first step in discovering the likelihood of a systemic reaction and the necessary precautions that may be prescribed. Walnut TC 3489 One of the most common causes WHOLE of allergic reactions to tree nuts.1,2 Walnut ALLERGEN • Estimated prevalence of walnut allergy in the general population is up to 0.7%.2 • Potentially life-threatening, increasing in prevalence and rarely outgrown.2,3 Associated with systemic Jug reactions2 r1 • Storage protein (2s albumin) 3,4 4 ALLERGEN • Heat and digestion stable COMPONENTS • Highly abundant in walnut Associated with local and Jug systemic reactions2 r3 • Lipid transfer protein (LTP)1,4 • Heat and digestion stable Knowing which Positive whole walnut with negative protein your patient is Jug r1 and Jug r3 results may be explained by sensitization to6: sensitized to can help • Other walnut storage proteins you develop a • Pollen proteins like profilin or PR-10 proteins management plan. • CCD (cross-reacting carbohydrate determinants) Cashew nut Brazil nut TC 2608 TC 2818 Allergy on the rise with increased Hidden allergen often found Cashew consumption in snack foods, Asian Brazil in cookies, insect repellent foods, baked goods, nut butters and and beauty products.12 pestos.8,9 • Extensive cross-reactivity within • Sensitized patients have a risk of experiencing the family can be expected.13 severe allergic reactions; the risk has been • Generally persists and is potentially reported to be even higher than for peanut life-threatening.14 allergic patients (74% vs. -

422 Part 180—Tolerances and Ex- Emptions for Pesticide
Pt. 180 40 CFR Ch. I (7–1–16 Edition) at any time before the filing of the ini- 180.124 Methyl bromide; tolerances for resi- tial decision. dues. 180.127 Piperonyl butoxide; tolerances for [55 FR 50293, Dec. 5, 1990, as amended at 70 residues. FR 33360, June 8, 2005] 180.128 Pyrethrins; tolerances for residues. 180.129 o-Phenylphenol and its sodium salt; PART 180—TOLERANCES AND EX- tolerances for residues. 180.130 Hydrogen Cyanide; tolerances for EMPTIONS FOR PESTICIDE CHEM- residues. ICAL RESIDUES IN FOOD 180.132 Thiram; tolerances for residues. 180.142 2,4-D; tolerances for residues. Subpart A—Definitions and Interpretative 180.145 Fluorine compounds; tolerances for Regulations residues. 180.151 Ethylene oxide; tolerances for resi- Sec. dues. 180.1 Definitions and interpretations. 180.153 Diazinon; tolerances for residues. 180.3 Tolerances for related pesticide chemi- 180.154 Azinphos-methyl; tolerances for resi- cals. dues. 180.4 Exceptions. 180.155 1-Naphthaleneacetic acid; tolerances 180.5 Zero tolerances. for residues. 180.6 Pesticide tolerances regarding milk, 180.163 Dicofol; tolerances for residues. eggs, meat, and/or poultry; statement of 180.169 Carbaryl; tolerances for residues. policy. 180.172 Dodine; tolerances for residues. 180.175 Maleic hydrazide; tolerances for resi- Subpart B—Procedural Regulations dues. 180.176 Mancozeb; tolerances for residues. 180.7 Petitions proposing tolerances or ex- 180.178 Ethoxyquin; tolerances for residues. emptions for pesticide residues in or on 180.181 Chlorpropham; tolerances for resi- raw agricultural commodities or proc- dues. essed foods. 180.182 Endosulfan; tolerances for residues. 180.8 Withdrawal of petitions without preju- 180.183 Disulfoton; tolerances for residues. -

Black Walnut Juglans Nigra
black walnut Juglans nigra Kingdom: Plantae FEATURES Division: Magnoliophyta The deciduous black walnut tree may grow to a Class: Magnoliopsida height of 150 feet and a diameter of five feet. The Order: Fagales trunk is straight, and the crown is rounded. The bark is thick, black and deeply furrowed. The pith in the Family: Juglandaceae twigs is chambered, that is, divided by partitions. ILLINOIS STATUS The bud is rounded at the tip, pale brown and hairy. The pinnately compound leaves have 15 to 23 common, native leaflets and are arranged alternately on the stem. © Guy Sternberg Each lance-shaped leaflet may be up to three and one-half inches long and one and one-half inches wide. The leaflet is toothed along the edges, yellow- green and smooth above and paler and hairy below. Leaves turn yellow in the fall. Male and female flowers are separate but located on the same tree. The male (staminate) flowers are arranged in yellow- green, hairy catkins, while the female (pistillate) flowers are in small spikes. Neither type of flower has petals. The spherical fruits are arranged in groups of one or two. Each green or yellow-green walnut may be up to two inches in diameter. The husk on the fruit is thick, while the nut is very hard, oval, dark brown and deeply ridged. The seed is sweet to the taste. tree in summer BEHAVIORS The black walnut may be found statewide in Illinois. ILLINOIS RANGE This tree grows in rich woodlands. The black walnut flowers in April and May when the leaves are partly grown. -

Food Oral Immunotherapy for Severe Food Allergies
FOOD ORAL IMMUNOTHERAPY FOR SEVERE FOOD ALLERGIES ALLISON FREEMAN MD Clinical Assistant Professor, University at Buffalo Division of Allergy/Immunology & Rheumatology GOALS • REVIEW UP TO DATE GUIDANCE ON THE DEVELOPMENT AND COSTS OF FOOD ALLERGY • KNOW HOW AND WHEN TO TEST FOR FOOD ALLERGY • DISCUSS FORMS OF THERAPY THAT ARE OR SOON WILL BE MARKETED • DISCUSS FORMS OF THERAPY THAT ARE NOW AVAILABLE AT UBMD PEDIATRIC ALLERGY & IMMUNOLOGY 1. SEVERE FOOD ALLERGY MAY BE PREVENTABLE • LEARNING EARLY ABOUT PEANUT ( DUTOIT ET AL , NEJM 2015) TAUGHT US THAT OUR 20 YEAR OLD STRATEGY OF DELAYING ALLERGENIC FOOD INTRODUCTION TO INFANTS WAS WRONG • THEY SHOWED IN A LARGE RANDOMIZED TRIAL THAT; • INFANTS OFTEN HAVE POSITIVE SKIN TESTING FOR ALLERGENIC FOODS BEFORE ORAL EXPOSURE, ESPECIALLY IF THEY HAVE SIGNIFICANT ECZEMA OR ONE CLINICAL FOOD ALLERGY ALREADY- THIS IS TERMED SENSITIZATION AND MAY BE A STAGE OF THE ALLERGIC MARCH WE CAN TURN AROUND • EARLY INTRODUCTION OF EGG, MILK AND POSSIBLY CASHEW ARE SHOWN TO LESSEN LATER ALLERGY • INFANTS WITH POSITIVE SKIN TESTS TO PEANUT ARE NOT ALL DANGEROUSLY ALLERGIC • INFANTS WITH PEANUT WHEAL < 8MM OFTEN TOLERATE CHALLENGE AND IF THEY CONTINUE TO EAT IT ROUTINELY THEY HAVE >85% RISK REDUCTION FOR PEANUT ALLERGY AT SCHOOL AGE 2. FOOD ALLERGY ONCE CONFIRMED CAN BE LIFE CHANGING • ALL CHILDREN DIAGNOSED BY CLINICAL REACTION WITH CONFIRMATORY TESTING, OR BY ORAL FOOD CHALLENGE NEED TO BE TAUGHT; • HOW TO READ A FOOD LABEL AND THAT FOODS ‘PROCESSED IN A FACTORY THAT ALSO PROCESSES ‘THEIR ALLERGEN IS A SERIOUS WARNING THAT THEY SHOULD FOLLOW • HOW TO USE THEIR EPINEPHRINE, AND WHAT CONSTITUTES ANAPHYLAXIS ( ANY MULTISYSTEM REACTION TO A KNOWN ALLERGEN FOR THAT PATIENT) • THAT EPINEPHRINE HELPS MOST WHEN DELIVERED EARLY, WHICH IS WHY THEY NEED TO CARRY IT EVERYWHERE ,KEEP IT IN DATE AND STORE IT CORRECTLY The Impact of Food Allergy Diagnoses • Risk of a severe anaphylaxis is just one of many concerns. -

Identification of Butternuts and Butternut Hybrids
Purdue University Purdue extension FNR-420-W & Natural Re ry sou Forestry and Natural Resources st rc re e o s F Identification of Butternuts and Butternut Hybrids Lenny Farlee1,3, Keith Woeste1, Michael Ostry2, James McKenna1 and Sally Weeks3 1 USDA Forest Service Hardwood Tree Improvement and Regeneration Center, Purdue University, 715 W. State Street, West Lafayette, IN, 47907 PURDUE UNIVERSITY 2 USDA Forest Service Northern Research Station, 1561 Lindig Ave. St. Paul, MN 55108 3 Department of Forestry and Natural Resources, Purdue University, 715 W. State Street, West Lafayette, IN, 47907 Introduction Butternut (Juglans cinerea), also known as white walnut, is a native hardwood related to black walnut (Juglans nigra) and other members of the walnut family. Butternut is a medium-sized tree with alternate, pinnately compound leaves that bears large, sharply ridged and corrugated, elongated, cylindrical nuts born inside sticky green hulls that earned it the nickname lemon-nut (Rink, 1990). The nuts are a preferred food of squirrels and other wildlife. Butternuts were collected and eaten by Native Americans (Waugh, 1916; Hamel and Chiltoskey, 1975) and early settlers, who also valued butternut for its workable, medium brown-colored wood (Kellogg, 1919), and as a source of medicine (Johnson, 1884), dyes (Hamel and Chiltoskey, 1975), and sap sugar. Butternut’s native range extends over the entire north- eastern quarter of the United States, including many states immediately west of the Mississippi River, and into Canada. Butternut is more cold-tolerant than black walnut, and it grows as far north as the Upper Peninsula of Michigan, New Brunswick, southern Quebec, and Figure 1.