Comparative Proteomic Analysis of Walnut (Juglans Regia L.) Pellicle Tissues Reveals the Regulation of Nut Quality Attributes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Some Chemical Composition of Walnut (Juglans Regia L.) Selections from Eastern Turkey
African Journal of Agricultural Research Vol. 5(17), pp. 2379-2385, 4 September, 2010 Available online at http://www.academicjournals.org/AJAR ISSN 1991-637X ©2010 Academic Journals Full Length Research Paper Some chemical composition of walnut (Juglans regia L.) selections from Eastern Turkey Ferhad Muradoglu1*, H. Ibrahim Oguz2, Kenan Yildiz3 and Hüdai Yilmaz1 1Department of Horticulture, Faculty of Agriculture, Universty of Yuzuncu Yil, 65080 Van, Turkey. 2Professional High School of Kahta, Adiyaman Universty, Adiyaman, Turkey. 3Department of Horticulture, Faculty of Agriculture, Universty of Gaziosmanpaa,Tokat, Turkey. Accepted 10 May, 2010 The aim of this study was to determine the chemical and mineral contents of eighteen walnut genotypes which were newly selected from Hizan (Bitlis) located in Eastern Anatolia. The protein, total fat, total oil (saturated and unsaturated oil) compositions and mineral contents were investigated. It was found that the average value for protein was 18.1% and for total fat was 58.2%. Saturated fatty acids composition values were less than the values of monounsaturated fatty acids composition and polyunsaturated fatty acids composition in all genotypes. Among the identified fatty acids, linoleic acid (50.58 - 66.60%) was the predominant fatty acid followed by oleic acid (14.88 - 28.71%) and linolenic acid (9.16 -16.42%) in all genotypes. The other fatty acids were found in trace contents. The minimum and maximum macronutrient contents of walnut were determined as mg100 g-1 for K (911.0 - 684.3), P (434.7 - 356.2), Ca (756.7 - 388.2), Mg (444.0 - 330.8) and Na (48.9 - 26.1) while minimum and maximum micronutrient contents of walnut were determined for Fe (6.6 - 4.3), Cu (2.8 - 1.8), Mn (5.7 - 2.7) and Zn (4.3 - 2.7). -

Growing Chinese Chestnuts in Missouri by Ken Hunt, Ph.D., Research Scientist, Center for Agroforestry, Blight
AGROFORESTRY IN ACTION University of Missouri Center for Agroforestry AF1007 - 2012 Growing Chinese Chestnuts in Missouri by Ken Hunt, Ph.D., Research Scientist, Center for Agroforestry, blight. In fact, the devastation caused by chestnut blight University of Missouri, Michael Gold, Ph.D., Associate Director, (Cryphonectria parasitica) stem cankers has reduced Center for Agroforestry, University of Missouri, William Reid, American chestnut from a major timber species to a rare Ph.D., Research and Extension Horticulturist, Kansas State Uni- understory tree often found cankered in sprout clumps. versity, & Michele Warmund, Ph.D., Professor of Horticulture, Major efforts are underway to restore the American Division of Plant Sciences, University of Missouri chestnut (see www.acf.org/). The Allegheny and Ozark chinkapins are multi-stem shrubs to small trees that hinese chestnut is an emerging new tree crop produce small tasty nuts and make interesting (but for Missouri and the Midwest. The Chinese blight susceptible) landscape trees that are also useful Cchestnut tree is a spreading, medium-sized tree for wildlife. with glossy dark leaves bearing large crops of nutri- tious nuts. Nuts are borne inside spiny burs that split open when nuts are ripe. Each bur contains one to three shiny, dark-brown nuts. Nuts are "scored" then micro- waved, roasted or boiled to help remove the leathery shell and papery seed coat, revealing a creamy or gold- en-colored meat. Chestnuts are a healthy, low-fat food ingredient that can be incorporated into a wide range of dishes – from soups to poultry stuffing, pancakes, muf- fins and pastries (using chestnut flour). Historically, demand for chestnuts in the United States has been highest in ethnic markets (European and Asian, for example) but as Americans search for novel and healthy food products, chestnuts are becoming more widely accepted. -
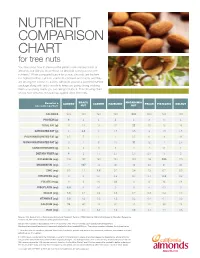
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Western Plant Diagnostic Network Newsletter
Western Plant Diagnostic Network Newsletter WPDN – First Detector Dear WPDN First Detectors, Two new pest problems Network News have been found in the WPDN region. The first, the fruit- piercing moth, has been found in Hawaii. It is a destructive moth with a broad host range. The second is a The newsletter for the disease called thousand cankers disease of walnut, which WPDN is caused by a fungus vectored by the walnut twig beetle. First Detector Community This disease has been found throughout the western U.S. Read more about these in the following articles. February 2010 Volume 3, Number 1 We now have over 3,750 First Detectors in the WPDN, achieved with several training sessions in Guam, Oregon, Contact us at the WPDN and California. Congratulations to all our WPDN First Regional Center: Detector Educators. Dr. Amanda Hodges, the NPDN Training and Education Chair, informs us that there is a Phone: 530 754 2255 new online Chilli Thrips module on the Online Training Fax: 530 754 7998 site. Chilli Thrips Email: [email protected] To access this new module, log on to the NPDN home page at: http://www.npdn.org/ Websites: https://www.wpdn.org https://www.npdn.org Published by the WPDN Regional Center Department of Plant Pathology University of California, Davis Click on this logo Editor: Richard W. Hoenisch ©Copyright Regents of the University of California All Rights Reserved And begin! A Fruit-Piercing Moth found in Hawaii Lepidoptera: Noctuidae Oraesia excavata Butler Personal Communication from Bernarr Kumashiro, entomologist with HDOA On December 28, 2009, William Haines, University of Hawaii, notified Hawaii Department of Agriculture (HDOA) of a new fruit-piercing moth in Hawaii. -
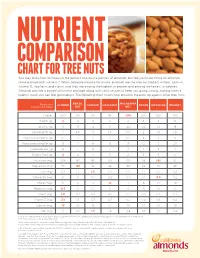
Chart for Tree Nuts
NUTRIENT COMPARISON CHART FOR TREE NUTS You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the highest in protein and among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a healthy snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT Calories 1602 190 160 180 200 200 160 190 Protein (g) 6 4 4 4 2 3 6 4 Total Fat (g) 14 19 13 17 22 20 13 18 Saturated Fat (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 Polyunsaturated Fat (g) 3.5 7 2 2 0.5 6 4 13 Monounsaturated Fat (g) 9 7 8 13 17 12 7 2.5 Carbohydrates (g) 6 3 9 5 4 4 8 4 Dietary Fiber (g) 4 2 1 3 2 3 3 2 Potassium (mg) 208 187 160 193 103 116 285 125 Magnesium (mg) 77 107 74 46 33 34 31 45 Zinc (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 Vitamin B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 Folate (mcg) 12 6 20 32 3 6 14 28 Riboflavin (mg) 0.3 0 0.1 0 0 0 0.1 0 Niacin (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 Vitamin E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.6 0.2 Calcium (mg) 76 45 13 32 20 20 30 28 Iron (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Almond Cashew Barfi Recipe
150 Jackson St, Petone T: 568 4149 E: [email protected] ALMOND CASHEW BARFI RECIPE Almond Walnut Cashew Barfi is a healthy, quality substitute for candy that your entire family will enjoy Ingredients Makes 24 pcs 1/2 c almonds 1/2 c water 1/2 c walnuts 1/2 tsp cardamom powder 1/2 c cashew nuts 1 Tbsp sliced almonds to garnish 1 1/4 c sugar Method 1. Dry grind the walnuts, cashews and almonds in a food processor. 2. Dry roast the grounded nuts in a frying pan on low medium heat. 3. Roast them just enough so that the nuts start to give off an aroma. It will take about 4 to 5 minutes. Remove from heat and set aside. 4. Put the sugar and water together in a saucepan on medium heat. Bring to a boil to make the 1 thread syrup or on the candy thermometer it should reach 230 degrees F. 5. Turn off the heat and stir in the cardamom powder. 6. Add the nuts to the syrup and mix, and then spread over a greased 8-inch plate. Note: don’t let the syrup cool off. It must be spread while still hot. 7. Wait a few minutes until barfi is set but still soft. 8. Then cut the barfi into any shape you like (such as square, diamond, triangle). 9. Garnish each piece of barfi with sliced almonds while the barfi is still soft. 10. Allow the barfi to cool for about an hour to dry and hold its shape. -

BAY AREA MENU Seasonal Items in Green Contains Gluten Vegan
BAY AREA MENU seasonal items in green contains gluten vegan Before placing your order, please inform your server if a person in your party has a food allergy. SEASONALS FALAFEL + FETA SPRING CHICKEN UMAMI GRAIN BOWL broccoli leaf, chopped organic mesclun, shredded organic quinoa + farro, romaine, roasted beets, kale, roasted zucchini + swiss chard, pea shoots, spicy broccoli, parsley, yellow squash + asparagus, red onion, spicy sunflower mint, local feta, baked basil, shredded carrots, seeds, roasted sesame falafel, lemon garlic chili parmesan crisps, roasted tofu, roasted portobello vinaigrette chicken, pesto vinaigrette mushrooms, miso ginger 460 cal $9 sesame dressing 480 cal $11.25 615 cal $10.25 GREENS SOUPS KALE CAESAR OMG OMEGA GUACAMOLE GREENS ORGANIC LENTIL CHICKPEA shredded kale, chopped organic arugula, organic organic mesclun, tomatoes, small 160 cal $3.50 romaine, tomatoes, shaved baby spinach, cucumbers, red onion, tortilla chips, large 240 cal $5.50 parmesan, parmesan crisps, tomatoes, basil, avocado, avocado, roasted chicken, roasted chicken, fresh lime nori furikake, roasted fresh lime squeeze, lime squeeze, caesar dressing steelhead, miso sesame cilantro jalapeño vinaigrette ginger dressing 430 cal $9.75 540 cal $10.75 550 cal $13.25 HUMMUS TAHINA RAD THAI SPICY SABZI BEVERAGES shredded kale, chopped organic arugula, organic organic baby spinach, $2.50 romaine, tomatoes, mesclun, bean sprouts, shredded kale, spicy red onion, cucumbers, carrots, shredded cabbage, quinoa, spicy broccoli, HIBISCUS LIME FRESCA pita chips, -

Wholesome and Organic
1 3231 Camino de los Coches #107 Carlsbad, CA. 92009 Tel: 760-230-9282 Instagram/FB: @bigfootnaturalcafe Organic – Vegan – Wholesome – GF Available Wholesome and Organic Pasta and Rice: Mac N’ Cheez (GF) $13.75 (GF macaroni, cauliflower, broccoli, carrots cooked with house broth and house made cashew cheez sauce) Cajun Dirty Mac (GF by request) $13.75 (GF mac with roasted veggies, mushrooms, black beans and tempeh bits, in a house made creamy Creole sauce packed with leafy greens) Woodland Rice Bowl (GF) $15.25 (Sprouted brown rice, grilled portabella mushrooms, black beans, roasted sweet potatoes, grilled zucchini and avocado, drizzled with house cheez sauce and mole sauce (chocolate free).) Upgrades: Add Avocado $2.50 or house made super GF bread $2.50 Hand Crafted Tacos and Burritos: Chili Guava infused Jackfruit Tacos (GF) $13.75 (2 tacos with chili-guava infused jackfruit, slaw, maple toasted coconut chips, avocado and cashew sauce. Served with a small side of sweet potato and black bean salad) Kung Pao Chickpea Tacos (GF) $13.75 (2 tacos with roasted kung pao chickpeas, cashews and shaved brussel sprouts , red slaw, maple toasted coconut chips, avocado and kung pao sauce. Served with a small side of sweet potato and black bean salad) Tiger Stripe Burrito $14.25 (GF Option Available) (Blackened tempeh, sprouted brown rice, sweet potatoes, black beans wrapped in a warm whole wheat tortilla and smothered in our house cheez and mole sauce. Served with a side salad.) Jaka Asada Burrito $15.25 (Rosemary potatoes, chili-guava infused jackfruit (Jaka), portabella mushrooms, soaked chickpeas, hemp hearts all tossed in our Mole made with Modern Times Blackhouse Coffee Stout, wrapped in a warm whole wheat tortilla and smothered in our house cheez sauce. -

U.S. EPA, Pesticide Product Label, , 07/22/2003
/40-/US Page 1 of 32 Oevrinol 50-OF Booklet OEV1035 draft revisions 5/23/03 Devrinol® 50-OF Selective Herbicide Dry Flowable For use on certain citrus, nuts, pome fruits, small fruits, stone fruits, vegetables and tobacco Active Ingredient: Napropamide (CAS No. 15299-99-7) ...................................................................................... 50% Other Ingredients: 50% Total: 100% Contains 50 Ibs. of active Ingredient per 100 Ibs. KEEP OUT OF REACH OF CHILDREN. CAUTION See additional precautionary statements and directions for use inside booklet. EPA Reg. No. 100-1035 EPA Est. ACCEPTED 0'- -n) ...... tiLiI; L In EPA Lettl."r Dated Made in U.S.A. JUL 22 2003 Ul)der the Federal Imectitide. fwadicide. IUtd Rtulcndcldle .4.ct WI lIDlendcd. [Dr th... .... U~lde SCP# t"ltW.erf'd und .. r 'ia'A, .•. rit~. ...lett>- 4 pounds Net Weight ( Page 2 of 32 Oevrinol 50-OF Booklet OEV1035 draft revisions 5/23/03 TABLE OF CONTENTS FIRST AID ....................................................................................................................................................... PRECAUTIONARY STATEMENTS ................................................................................................................ Hazards To Humans And Domestic Animals ........................................................................................ .. Personal Protective Equipment. .............................................................................................................. Environmental Hazards .......................................................................................................................... -

Food Oral Immunotherapy for Severe Food Allergies
FOOD ORAL IMMUNOTHERAPY FOR SEVERE FOOD ALLERGIES ALLISON FREEMAN MD Clinical Assistant Professor, University at Buffalo Division of Allergy/Immunology & Rheumatology GOALS • REVIEW UP TO DATE GUIDANCE ON THE DEVELOPMENT AND COSTS OF FOOD ALLERGY • KNOW HOW AND WHEN TO TEST FOR FOOD ALLERGY • DISCUSS FORMS OF THERAPY THAT ARE OR SOON WILL BE MARKETED • DISCUSS FORMS OF THERAPY THAT ARE NOW AVAILABLE AT UBMD PEDIATRIC ALLERGY & IMMUNOLOGY 1. SEVERE FOOD ALLERGY MAY BE PREVENTABLE • LEARNING EARLY ABOUT PEANUT ( DUTOIT ET AL , NEJM 2015) TAUGHT US THAT OUR 20 YEAR OLD STRATEGY OF DELAYING ALLERGENIC FOOD INTRODUCTION TO INFANTS WAS WRONG • THEY SHOWED IN A LARGE RANDOMIZED TRIAL THAT; • INFANTS OFTEN HAVE POSITIVE SKIN TESTING FOR ALLERGENIC FOODS BEFORE ORAL EXPOSURE, ESPECIALLY IF THEY HAVE SIGNIFICANT ECZEMA OR ONE CLINICAL FOOD ALLERGY ALREADY- THIS IS TERMED SENSITIZATION AND MAY BE A STAGE OF THE ALLERGIC MARCH WE CAN TURN AROUND • EARLY INTRODUCTION OF EGG, MILK AND POSSIBLY CASHEW ARE SHOWN TO LESSEN LATER ALLERGY • INFANTS WITH POSITIVE SKIN TESTS TO PEANUT ARE NOT ALL DANGEROUSLY ALLERGIC • INFANTS WITH PEANUT WHEAL < 8MM OFTEN TOLERATE CHALLENGE AND IF THEY CONTINUE TO EAT IT ROUTINELY THEY HAVE >85% RISK REDUCTION FOR PEANUT ALLERGY AT SCHOOL AGE 2. FOOD ALLERGY ONCE CONFIRMED CAN BE LIFE CHANGING • ALL CHILDREN DIAGNOSED BY CLINICAL REACTION WITH CONFIRMATORY TESTING, OR BY ORAL FOOD CHALLENGE NEED TO BE TAUGHT; • HOW TO READ A FOOD LABEL AND THAT FOODS ‘PROCESSED IN A FACTORY THAT ALSO PROCESSES ‘THEIR ALLERGEN IS A SERIOUS WARNING THAT THEY SHOULD FOLLOW • HOW TO USE THEIR EPINEPHRINE, AND WHAT CONSTITUTES ANAPHYLAXIS ( ANY MULTISYSTEM REACTION TO A KNOWN ALLERGEN FOR THAT PATIENT) • THAT EPINEPHRINE HELPS MOST WHEN DELIVERED EARLY, WHICH IS WHY THEY NEED TO CARRY IT EVERYWHERE ,KEEP IT IN DATE AND STORE IT CORRECTLY The Impact of Food Allergy Diagnoses • Risk of a severe anaphylaxis is just one of many concerns. -

Comparative Analysis of Mineral Elements and Essential Amino Acids Compositions in Juglans Sigillata and J
ZhaiAvailable M. et al./ online: Not Bot www.notulaebotanicae.ro Horti Agrobo, 2014, 42(1):36-42 Print ISSN 0255-965X; Electronic 1842-4309 Not Bot Horti Agrobo , 2014, 42(1):36-42 Comparative Analysis of Mineral Elements and Essential Amino Acids Compositions in Juglans sigillata and J. regia Walnuts Kernels Meizhi ZHAI*, Zhenyuan WANG, Dan WANG, Jing XU, Guanzhao SHI Northwest Agriculture & Forestry University, Walnut Research Center, College of Forestry, Yangling, Shaanxi, China; [email protected] (*corresponding author) Abstract Walnut kernel is famous for its high nutritional and economic values. The kernel is usually considered to be a good source of minerals and essential amino acids. In this paper, mineral elements (calcium, magnesium, iron, manganese, copper, and zinc) and essential amino acids (phenylalanine, valine, threonine, isoleucine, leucine, methionine, and lysine) compositions of kernels from 11 kinds of walnuts ( Juglans sigillata ) and 17 kinds of walnuts ( Juglans regia ) originated from China were determined by ICP-MS and HPLC, respectively. The order of nutritive mineral elements depending on their content (mg/100g) of samples was Mg> Ca> Zn> Mn> Fe> Cu in J. regia , while the order in J. sigillata was Mg> Ca> Mn> Fe > Zn > Cu. For essential amino acids, the order depending on the content (mg/g) of the essential amino acids in J. regia samples was leucine> isoleucine> valine> phenylalanine> lysine> threonine> methionine, while the order in J. sigillata was leucine> isoleucine> lysine> phenylalanine> valine> threonine> methionine. The kernels of walnuts ( J. regia and J. regia ) are good sources of health foods and dietary supplements. ‘Y029’ in Juglans sigillata and ‘XJ004’ in Juglans regia provided the best profiles of mineral elements and essential amino acids in comparison to others. -

Juglans Regiaregia Carpathiancarpathian Walnut,Walnut, Persianpersian Walnutwalnut
JuglansJuglans regiaregia CarpathianCarpathian walnut,walnut, PersianPersian walnutwalnut SEASONAL COLOURS jan feb mar apr mei jun jul aug sep okt nov dec TYPES OF PLANTING Tree types: standard trees, half-stem trees, multi-stemmed trees, trees for climbing, characteristic trees, fruit trees, woodland planting stock | Topiary on stem: vase, multi-stem umbrella USE Location: park, central reservation, roof garden, large garden, traffic areas, industrial zones, countryside, ecological zone | Pavement: none | Planting concepts: food forest, Landscape planting, Prairie planting CHARACTERISTICS Crown shape: flattened spherical | Crown structure: semi-open | Height: 20 - 30 m | Width: 20 - 30 m | Winter hardiness zone: 5B - 9B ASPECTS Wind: tolerant to wind | Soil: loess, sabulous clay, light clay, sand, loamy soil | Nutrient level: moderately rich in nutrients, rich in nutrients | Soil moisture level: dry, moist | Light requirements: sun | pH range: neutral, alkaline | Host plant/forage plant: birds, small mammals, humans | Extreme environments: tolerates air pollution PLANTKENMERKEN Flowers: catkins, pendulous | Flower colour: yellow-green | Flowering period: April - May | Leaf colour: buds bronze, green, underside pale green | Leaves: deciduous, ovoid, big, leathery , composite | Autumn colour: yellow-brown | Fruits: striking, edible, large, nut, drupe | Fruit colour: brown, green | Bark colour: pale grey | Bark: deeply furrowed, smooth, later on rough | Twig colour: pale grey | Twigs: bare | Root system: deep, fine roots, tap root Powered by TCPDF (www.tcpdf.org).