Brazil Nut: Nutritional Benefits from a Unique Combination of Antioxidants
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
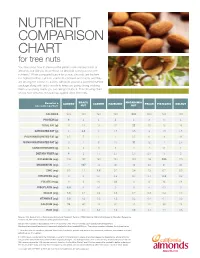
Nutrient Comparison Chart
NUTRIENT COMPARISON CHART for tree nuts You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a satisfying snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT CALORIES 1602 190 160 180 200 200 160 190 PROTEIN (g) 6 4 4 4 2 3 6 4 TOTAL FAT (g) 14 19 13 17 22 20 13 19 SATURATED FAT (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 POLYUNSATURATED FAT (g) 3.5 7 2 2 0.5 6 4 13 MONOUNSATURATED FAT (g) 9 7 8 13 17 12 7 2.5 CARBOHYDRATES (g) 6 3 9 5 4 4 8 4 DIETARY FIBER (g) 4 2 1.5 2.5 2.5 2.5 3 2 POTASSIUM (mg) 208 187 160 193 103 116 285 125 MAGNESIUM (mg) 77 107 74 46 33 34 31 45 ZINC (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 VITAMIN B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 FOLATE (mcg) 12 6 20 32 3 6 14 28 RIBOFLAVIN (mg) 0.3 0 0.1 0 0 0 0.1 0 NIACIN (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 VITAMIN E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.7 0.2 CALCIUM (mg) 76 45 13 32 20 20 30 28 IRON (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

Pistachio and Walnut Development Project TCP/KYR/3203 (D)
State Agency of Forestry and Environmental Protection, Kyrgyz Republic Pistachio and Walnut Development Project TCP/KYR/3203 (D) Hafiz Muminjanov Plant Production & Protection Officer Food and Agriculture Organization of the United Nations Sub-regional Office for Central Asia (FAOSEC) Agriculture in Kyrgyz Republic • Cereals - 1.5 mln MT, • including wheat 0.7 mln. MT • Potato - 1. 3 mln. MT • Vegetables - 0.8 mln. MT • Fruits -200 000 MT • Grapes - 10 000 MT 1 Why pistachio and walnut? Total Plantation Production, Yield, Area, ha Area, ha MT kg/ha Pistachio 57 000 12000 1 000 15-20 Walnut 47 000 - 900-1 900 20-40 Agriculture growth vs Overall Growth Kyrgyz Republic - Ag. Growth Vs. Overall Growth 160 Total GDP (1990=100) 140 AgGDP (1990=100) Total GDP (2002=100) AgGDP (2002=100) 120 100 AgGDP 80 (1990=100) Total GDP 60 (1990=100) 40 2 Wheat Grain Trade Balance ('000 tons) 1,200 1,000 800 600 Import 400 Domestic Production 200 - 2003 2004 2005 2006 2007 Trade Balance Values Dry Nuts Vs. Agriculture (2001 = 100) 600 Dry Nuts 400 200 0 2001 2002 2003 2004 2005 2006 - 200 - 400 Agriculture - 600 3 Why pistachio and walnut? • Central Asia – centre of origin & diversity of nuts • Large area of forest plantations • Suitable climatic conditions (dry summer) • Suitable socio-economic environment • Organic forests 4 Why pistachio and walnut? • Absence of market orientation in the past • Current varieties not valued for international market • No management done (cutting, pruning, etc.) • Density is high, pistachio male/female ratio not regulated • Yield is low • History of quality produce • International standards (USA, France, Chile, Australia) • Demand increase • Potential for export • Increasing farmers income & Livelihood improvement 5 Why pistachio and walnut? Development of the nut fruit sector has a high potential in improving livelihood and food security due the high international demand… Past and Related Work • Tien Shan Ecosystem Development Project (IFAD; GEF; WB-Bio Carbon Fund; Japanese Government). -
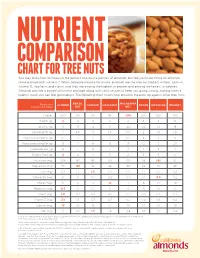
Chart for Tree Nuts
NUTRIENT COMPARISON CHART FOR TREE NUTS You may know how to measure the perfect one-ounce portion of almonds, but did you know those 23 almonds come packed with nutrients? When compared ounce for ounce, almonds are the tree nut highest in fiber, calcium, vitamin E, riboflavin and niacin, and they are among the highest in protein and among the lowest in calories. Almonds provide a powerful nutrient package along with tasty crunch to keep you going strong, making them a healthy snack you can feel good about. The following chart shows how almonds measure up against other tree nuts. BRAZIL MACADAMIA Based on a ALMOND CASHEW HAZELNUT PECAN PISTACHIO WALNUT one-ounce portion1 NUT NUT Calories 1602 190 160 180 200 200 160 190 Protein (g) 6 4 4 4 2 3 6 4 Total Fat (g) 14 19 13 17 22 20 13 18 Saturated Fat (g) 1 4.5 3 1.5 3.5 2 1.5 1.5 Polyunsaturated Fat (g) 3.5 7 2 2 0.5 6 4 13 Monounsaturated Fat (g) 9 7 8 13 17 12 7 2.5 Carbohydrates (g) 6 3 9 5 4 4 8 4 Dietary Fiber (g) 4 2 1 3 2 3 3 2 Potassium (mg) 208 187 160 193 103 116 285 125 Magnesium (mg) 77 107 74 46 33 34 31 45 Zinc (mg) 0.9 1.2 1.6 0.7 0.4 1.3 0.7 0.9 Vitamin B6 (mg) 0 0 0.1 0.2 0.1 0.1 0.3 0.2 Folate (mcg) 12 6 20 32 3 6 14 28 Riboflavin (mg) 0.3 0 0.1 0 0 0 0.1 0 Niacin (mg) 1.0 0.1 0.4 0.5 0.7 0.3 0.4 0.3 Vitamin E (mg) 7.3 1.6 0.3 4.3 0.2 0.4 0.6 0.2 Calcium (mg) 76 45 13 32 20 20 30 28 Iron (mg) 1.1 0.7 1.7 1.3 0.8 0.7 1.1 0.8 Source: U.S. -

American Pecan
THE AMERICAN PECAN CRACKING OPEN THE NUTRITION STORY OF OUR NATIVE NUT Scientific evidence suggests but does not prove YOU THINK OF THEM FOR PIE. that eating 1.5 ounces per day of most nuts, YOU ADORE THEM IN PRALINES. such as pecans, as part of a diet low in saturated BUT DID YOU KNOW PECANS fat and cholesterol may reduce the risk of ARE ACTUALLY A NUTRITION heart disease.* POWERHOUSE? U.S. Food and Drug Administration Don’t be fooled by their rich, buttery texture and naturally sweet taste, pecans are extremely *One serving of pecans (28g) contains 18g of unsaturated fat and only 2g of saturated fat. nutrient dense. The macronutrient profile of pecans is appealing to many people: Pecans contain the same beneficial unsaturated protein (3 grams), carbohydrate (4 grams) and fat (20 grams). fats that are found in other nuts, and nearly two decades of research suggests that nuts, including A handful of pecans – about 19 halves – is a good source of fiber, pecans, may help promote heart health. thiamin, and zinc, and an excellent source of copper and manganese – a mineral that’s essential for metabolism and In each 1-ounce serving of raw pecans you’ll get bone health. 12 grams of “good” monounsaturated fat, with zero cholesterol or sodium.1 Compared to other To top it off, pecans contain polyphenols, specifically flavonoids nuts, pecans are among the lowest in carbs and – which are the types of bioactive compounds found in brightly highest in fiber. colored produce.2 So think of pecans as a supernut. -

Juglans Nigra Juglandaceae L
Juglans nigra L. Juglandaceae LOCAL NAMES English (walnut,American walnut,eastern black walnut,black walnut); French (noyer noir); German (schwarze Walnuß); Portuguese (nogueira- preta); Spanish (nogal negro,nogal Americano) BOTANIC DESCRIPTION Black walnut is a deciduous tree that grows to a height of 46 m but ordinarily grows to around 25 m and up to 102 cm dbh. Black walnut develops a long, smooth trunk and a small rounded crown. In the open, the trunk forks low with a few ascending and spreading coarse branches. (Robert H. Mohlenbrock. USDA NRCS. The root system usually consists of a deep taproot and several wide- 1995. Northeast wetland flora: Field office spreading lateral roots. guide to plant species) Leaves alternate, pinnately compound, 30-70 cm long, up to 23 leaflets, leaflets are up to 13 cm long, serrated, dark green with a yellow fall colour in autumn and emits a pleasant sweet though resinous smell when crushed or bruised. Flowers monoecious, male flowers catkins, small scaley, cone-like buds; female flowers up to 8-flowered spikes. Fruit a drupe-like nut surrounded by a fleshy, indehiscent exocarp. The nut has a rough, furrowed, hard shell that protects the edible seed. Fruits Bark (Robert H. Mohlenbrock. USDA NRCS. 1995. Northeast wetland flora: Field office produced in clusters of 2-3 and borne on the terminals of the current guide to plant species) season's growth. The seed is sweet, oily and high in protein. The bitter tasting bark on young trees is dark and scaly becoming darker with rounded intersecting ridges on maturity. BIOLOGY Flowers begin to appear mid-April in the south and progressively later until early June in the northern part of the natural range. -

Tree Nut Allergen Component Testing Brochure
Walnut, cashew, and Brazil nut allergen component testing Jug r1 Walnut, Jug cashew, and r3 Brazil nut allergens by Ana o3 the numbers Ber e1 Detect sensitizations to the whole nut to create personalized management plans for your patients. Allergen component testing Measurement of specific IgE by blood test that provides objective assessment of sensitization to the whole nut protein is the first step in discovering the likelihood of a systemic reaction and the necessary precautions that may be prescribed. Walnut TC 3489 One of the most common causes WHOLE of allergic reactions to tree nuts.1,2 Walnut ALLERGEN • Estimated prevalence of walnut allergy in the general population is up to 0.7%.2 • Potentially life-threatening, increasing in prevalence and rarely outgrown.2,3 Associated with systemic Jug reactions2 r1 • Storage protein (2s albumin) 3,4 4 ALLERGEN • Heat and digestion stable COMPONENTS • Highly abundant in walnut Associated with local and Jug systemic reactions2 r3 • Lipid transfer protein (LTP)1,4 • Heat and digestion stable Knowing which Positive whole walnut with negative protein your patient is Jug r1 and Jug r3 results may be explained by sensitization to6: sensitized to can help • Other walnut storage proteins you develop a • Pollen proteins like profilin or PR-10 proteins management plan. • CCD (cross-reacting carbohydrate determinants) Cashew nut Brazil nut TC 2608 TC 2818 Allergy on the rise with increased Hidden allergen often found Cashew consumption in snack foods, Asian Brazil in cookies, insect repellent foods, baked goods, nut butters and and beauty products.12 pestos.8,9 • Extensive cross-reactivity within • Sensitized patients have a risk of experiencing the family can be expected.13 severe allergic reactions; the risk has been • Generally persists and is potentially reported to be even higher than for peanut life-threatening.14 allergic patients (74% vs. -

Pecan Halves & Pieces
Fort Bend 4-H Pecan Sale 1402 Band Rd . Suite 100 Rosenberg,TX 77471 281-342-3034 PECAN HALVES & PIECES I lb. Bag Pecan Halves—$10.00 I lb. Bag Pecan Pieces—$9.00 3 lb. Box Pecan Halves—$29.00 3 lb. Box Pecan Pieces—$28.00 FLAVORED PECANS & GIFT BASKETS 1 lb. TX Basket Mixed Choc. Pecans 12 oz Praline Frosted Pecans $20.00 $8.00 12 oz Milk Choc. Pecans $7.00 1 lb. Pecan Sampler $14.00 SPECIALTY NUTS 1 lb. Bag Hot & Spicy Peanuts 1 lb. Bag Walnut Halves 12 oz Bag Chocolate Peanuts $4.00 $9.00 $5.00 1 lb. Bag R/S Redskin Peanuts $4.00 MIXES 1 lb. Bag Cran-Slam Mix 1 lb. Bag Fiesta Mix 1 lb. Bag Hunter’s Mix $7.00 $4.00 $6.00 Dried cranberries, walnut pieces, Bbq corn sticks, taco sesame Cashews, cocktail peanuts, roasted and salted almonds, sticks, nacho cheese pea- sesame sticks, sesame roasted and salted sunflower nuts, hot & spicy peanuts seeds, natural almonds, fan- seeds, diced pineapple, black rai- cy pecan halves, peanut oil, sins, roasted and salted pumpkin salt seeds 1 lb. Bag Mountain Mix 1 lb. Texas Deluxe Mix 1 lb. Trash Mix $6.00 $9.00 $4.00 Roasted and salted cocktail Pecan halves, cashews, nat. Cocktail peanuts, hot & spicy pea- peanuts, roasted and salted Almonds, brazil nuts, pea- nuts, pretzels, sesame sticks almonds, roasted and salted nut oil, salt. cashews, raisins, and m&m's WE delicious reach _______________________________________, Would ________________________________________. Turn 15. 14. 13. 12. 11. -

Black Walnut Juglans Nigra
black walnut Juglans nigra Kingdom: Plantae FEATURES Division: Magnoliophyta The deciduous black walnut tree may grow to a Class: Magnoliopsida height of 150 feet and a diameter of five feet. The Order: Fagales trunk is straight, and the crown is rounded. The bark is thick, black and deeply furrowed. The pith in the Family: Juglandaceae twigs is chambered, that is, divided by partitions. ILLINOIS STATUS The bud is rounded at the tip, pale brown and hairy. The pinnately compound leaves have 15 to 23 common, native leaflets and are arranged alternately on the stem. © Guy Sternberg Each lance-shaped leaflet may be up to three and one-half inches long and one and one-half inches wide. The leaflet is toothed along the edges, yellow- green and smooth above and paler and hairy below. Leaves turn yellow in the fall. Male and female flowers are separate but located on the same tree. The male (staminate) flowers are arranged in yellow- green, hairy catkins, while the female (pistillate) flowers are in small spikes. Neither type of flower has petals. The spherical fruits are arranged in groups of one or two. Each green or yellow-green walnut may be up to two inches in diameter. The husk on the fruit is thick, while the nut is very hard, oval, dark brown and deeply ridged. The seed is sweet to the taste. tree in summer BEHAVIORS The black walnut may be found statewide in Illinois. ILLINOIS RANGE This tree grows in rich woodlands. The black walnut flowers in April and May when the leaves are partly grown. -

Identification of Butternuts and Butternut Hybrids
Purdue University Purdue extension FNR-420-W & Natural Re ry sou Forestry and Natural Resources st rc re e o s F Identification of Butternuts and Butternut Hybrids Lenny Farlee1,3, Keith Woeste1, Michael Ostry2, James McKenna1 and Sally Weeks3 1 USDA Forest Service Hardwood Tree Improvement and Regeneration Center, Purdue University, 715 W. State Street, West Lafayette, IN, 47907 PURDUE UNIVERSITY 2 USDA Forest Service Northern Research Station, 1561 Lindig Ave. St. Paul, MN 55108 3 Department of Forestry and Natural Resources, Purdue University, 715 W. State Street, West Lafayette, IN, 47907 Introduction Butternut (Juglans cinerea), also known as white walnut, is a native hardwood related to black walnut (Juglans nigra) and other members of the walnut family. Butternut is a medium-sized tree with alternate, pinnately compound leaves that bears large, sharply ridged and corrugated, elongated, cylindrical nuts born inside sticky green hulls that earned it the nickname lemon-nut (Rink, 1990). The nuts are a preferred food of squirrels and other wildlife. Butternuts were collected and eaten by Native Americans (Waugh, 1916; Hamel and Chiltoskey, 1975) and early settlers, who also valued butternut for its workable, medium brown-colored wood (Kellogg, 1919), and as a source of medicine (Johnson, 1884), dyes (Hamel and Chiltoskey, 1975), and sap sugar. Butternut’s native range extends over the entire north- eastern quarter of the United States, including many states immediately west of the Mississippi River, and into Canada. Butternut is more cold-tolerant than black walnut, and it grows as far north as the Upper Peninsula of Michigan, New Brunswick, southern Quebec, and Figure 1. -

Pecan Varieties for Georgia Orchards
Pecan Varieties for Georgia Orchards By Lenny Wells and Patrick Conner, Department of Horticulture stablishing a new orchard or renovating an older to consider when selecting a variety are pollination Eorchard is an exciting time for a pecan grower type, disease resistance, alternate bearing potential, because it provides an opportunity to significantly precocity (the bearing age of the tree), harvest date, improve the productivity of the farm. However, pollination type, nut size and nut quality. choosing which cultivars to plant can be one of the most difficult decisions a grower has to make. Pecan Cultivar Characteristics cultivars vary widely in yield potential, nut quality, Pollination Type date of maturity, tree form and resistance to insects Pecan trees produce separate male (catkins) and and diseases. Problems encountered due to a poor female (nut cluster) flowers on the same tree. Basic choice in cultivars can be difficult or impossible to flowering timing follows one of two patterns. Type I correct through cultural methods, and may lead to the (protandrous) cultivars release pollen from the catkins time and expense of re-planting. It is vital that new first, and later the stigmas become receptive. Type cultivars be chosen with care since a productive pecan II (protogynous) cultivars have stigmas that become orchard can last for generations. receptive first and the pollen is shed after the flowers Growers naturally want to know what the “best” have been fertilized. In natural pecan stands about one variety is to plant. Blanket recommendations should half of the trees will be Type I and the other half Type not be made because every orchard situation is II. -

U.S. Pecan Market Insight
AGRICULTURAL FINANCE U.S. Pecan Market Insight April 15, 2021 Executive Summary The U.S. pecan industry has endured trade wars, hurricanes, and import competition in recent years. However, we still expect U.S. growers will likely benefit from positive long-term fundamentals. U.S. commercial pecan producers have increased yields and profitability by converting native orchards to more improved tree varieties. Additionally, the U.S. already accounts for a significant share of global production. And as pecans’ popularity continues to rise among a growing global middle class, increasingly productive U.S. growers are well-positioned to capitalize on expanding demand. MetLife Investment Management 2 Improving Productivity The composition of the U.S. pecan industry Figure 1 | Average Pecan Yield (2016-20, in-shell, pounds per acre) has shifted significantly over the last ten years.1 Historically, pecans were produced 2500 in native orchards in Georgia and Texas, which generate highly variable output due to 2000 alternate bearing. In the early 2000s, more 1500 modern orchards began to appear in Arizona and New Mexico. Lower rainfall in the 1000 region forced growers to invest in irrigation 500 and more actively manage their orchards, compared to native groves. Growers in 0 the U.S. Southwest planted improved New Mexico Arizona Georgia Texas tree varieties and optimized management Source: USDA, MIM practices like spacing, irrigation systems, and harvest techniques. The climate also tends to be more stable in that region allowing Figure 2 | U.S. Pecan Production by Tree Variety farmers to better control disease. As a (mil. lbs.) result, Arizona and New Mexico growers 450 have achieved higher yields than those in 400 Georgia—typically the leading U.S. -

Harvesting and Storing Your Home Orchard's Nut Crop
PUBLICATION 8005 Harvesting and Storing Your Home Orchard’s Nut Crop: Almonds, Walnuts, Pecans, Pistachios, and Chestnuts UNIVERSITY OF CALIFORNIA ED PERRY, University of California Cooperative Extension Farm Advisor, Division of Agriculture Stanislaus County; G. STEVEN SIBBETT, University of California Cooperative Extension and Natural Resources Farm Advisor, Tulare County. http://danrcs.ucdavis.edu he quality and quantity of your home orchard’s nut crop depend on the harvest- Ting, processing, and storage techniques that you use. This publication offers advice for handling almonds, chestnuts, pecans, pistachios, and walnuts. GENERAL TIPS When to Harvest Harvest all types of nuts as soon as they are ready. Late harvesting reduces crop volume, lowers nut quality, and shortens storage life. In California, harvest timing varies somewhat with location. The earliest harvest generally occurs in the southern San Joaquin Valley, followed by the central San Joaquin Valley, the Sacramento Valley, and finally the coastal valleys. An interval of about seven days separates the begin- ning dates for harvest in each succeeding district. Harvesting To harvest nuts (except chestnuts), knock or shake them from their trees with long wooden, fiberglass, or plastic (PVC) poles. You can knock almonds from the trees using rubber mallets or poles that are available from farm supply stores. CAUTION: When harvesting nuts with a pole, use extreme caution. Any contact between the pole and power lines will result in serious injury or death. Do not use aluminum or other metal poles to harvest nuts: these are especially hazardous around power lines. Loosened nuts often follow the pole to the ground, striking the person holding the pole.