The Transporter Associated with Antigen Processing: a Key Player in Adaptive Immunity
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Antigen Presentation by Dendritic Cells and Their Significance in Antineoplastic Immunotherapy
in vivo 18: 81-100 (2004) Antigen Presentation by Dendritic Cells and their Significance in Antineoplastic Immunotherapy BELA BODEY1,2, STUART E. SIEGEL1,2 and HANS E. KAISER3 1Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA; 2Childrens’ Center for Cancer and Blood Diseases, Childrens’ Hospital Los Angeles, Los Angeles, CA; 3Department of Pathology, School of Medicine, University of Maryland, Baltimore, MD, U.S.A. and Department of General and Experimental Pathology, University of Vienna, Vienna, Austria Abstract. Dendritic cells (DCs) are present in essentially every derived peptides on the surface of major histocompatibility mammalian tissue, where they operate at the interface of innate complex (MHC) molecules and acquire the cellular specialization and acquired immunity by recognizing pathogens and presenting to select and activate naive antigen-specific T lymphocytes. pathogen-derived peptides to T lymphocytes. According to the Immunotherapeutic ideas are based on the ability of the research group of Shortman (1-9), experimental results suggest a mammalian immune system to recognize neoplastically "dual" DC differentiation model, demonstrating the existence of transformed cells. Immunotherapy of human neoplasms has both myeloid-derived (with characteristic IF: CD11b+, CD11c+, always represented a very attractive fourth-modality therapeutic CD8alpha- and DEC205+) and lymphoid-derived DCs (showing approach, especially in light of the many shortcomings of CD11b-, CD11c-, CD8alpha+ and DEC205+ IF). DCs, including conventional surgical, radiation and chemotherapies in the interdigitating cells (IDCs) and Langerhans cells (LCs), are management of neoplastically transformed cells. The cancer characterized by dendritic morphology, high migratory mobility and vaccine approach to therapy is based on the notion that the are the most effective, "professional" cells for antigen presentation immune system could possibly mount a rejection strength response in primary immune responses. -

Processing and Presentation of Glycoproteins in the MHC Class I and II Antigen Presentation Pathways Denise Golgher, Tim Elliott and Mark Howarth
CHAPTER 11 Processing and Presentation of Glycoproteins in the MHC Class I and II Antigen Presentation Pathways Denise Golgher, Tim Elliott and Mark Howarth Abstract D8+ and CD4+ T cells are stimulated by peptides bound to MHC class I and II respec- tively. The processing pathways for the generation of class I or class II binding peptides C are specialized in surveying different intracellular compartments. While class I peptide loading occurs in the ER, class II loading occurs in the endocytic compartments. Many of the peptides generated in either compartment are derived from glycoproteins from normal or malignant cells or from intracellular or extracellular pathogens. In a given polypeptide there are potentially many T cell epitopes but only a few actually bind to the MHC molecules and generate a T cell response, while many others are cryptic. The carbohydrate moiety present in glycoproteins has been shown to influence the antigen processing pathway and generation of T cell epitopes in different ways. It can stabilize a certain three-dimensional conformation of the glycoprotein determining which sites are more accessible to proteases, it can hinder the access of proteases or block certain sites, it can target exogenous antigen to different antigen presenting cells and it can also be part of the epitope that will stimulate T cells. Changes in glycosylation patterns are common in malignancies, age and pathological mechanisms. These changes have the potential to alter the hierarchy of peptides that will bind to MHC molecules and induce a T cell response that would otherwise be cryptic, causing the onset of undesirable immune responses as is the case for autoimmune processes. -

Antigen Processing (Major Hbitocompatlbility Complex/Class I Moecules/Lymphokines) YOUNG YANG, JAMES B
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 4928-4932, June 1992 Immunology Proteasomes are regulated by interferon y: Implications for antigen processing (major hbitocompatlbility complex/class I moecules/lymphokines) YOUNG YANG, JAMES B. WATERS, KLAUS FROH, AND PER A. PETERSON Department of Immunology, The Scripps Research Institute, La Jolla, CA 92037 Communicated by Frank J. Dixon, February 27, 1992 ABSTRACT Class I major histocompatibility complex strengthened by the findings, presented in this communica- (MHC) molecules present antigenic peptides of cytoplasmic tion, that several proteasomal subunits, including MHC- origin to T cells. As the lengths ofthese peptides seem stried encoded subunits, are regulated by interferon y (IFN--y) and to eight or nine amino acids, an unusual proteolytic system that the incorporation of several more subunits into protea- must play a role in antigen processing. Proteasomes, a major somes appears to depend on the expression of the MHC- extralysosomal proteolytic system, are responsible for the encoded proteasomal subunits. Moreover, the pattern of degradation of cytoplasmic proteins. We demonstrate that expression of IFN-y-regulated subunits suggests complexi- several proteasomal subunits, including MHC-encoded sub- ties in the regulation of proteasomes with respect to its units, are regulated by interferon y. These data and the finding subunit composition, subcellular localization, and its incor- that MHC-encoded and other interferon -regulated protea- poration into larger ubiquitin-related proteolytic complexes. somal subunits are uniquely associated with proteasomes Possible functions for the MHC-encoded and IFN-y- strongly suggest that the immune system has recruited protea- regulated proteasomal subunits in antigen processing are somes for antigen processing. -

Cytoplasmic Proteolysis Antigens on MHC Class II Is Dependent On
Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis This information is current as of September 28, 2021. Paushali Mukherjee, Aadish Dani, Sumeena Bhatia, Nagendra Singh, Alexander Y. Rudensky, Anna George, Vineeta Bal, Satyajit Mayor and Satyajit Rath J Immunol 2001; 167:2632-2641; ; doi: 10.4049/jimmunol.167.5.2632 Downloaded from http://www.jimmunol.org/content/167/5/2632 References This article cites 51 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/167/5/2632.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2001 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis1 Paushali Mukherjee,* Aadish Dani,† Sumeena Bhatia,* Nagendra Singh,* Alexander Y. -
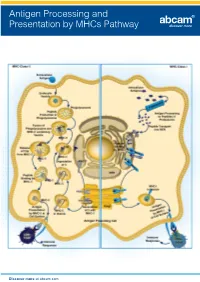
Antigen Processing and Presentation by Mhcs Pathway
Antigen Processing and Presentation by MHCs Pathway Discover more at abcam.com Antigen Processing and Presentation by MHCs Pathway Related antibodies Product This image shows MHC Class II highlights Product Clonality Applications Host Species Reactivity Product code expressing cells in formalin fixed and paraffin embedded human lymph MHC class I antibody [EP1395Y] M Flow Cyt, ICC/IF, IHC-P, IP, WB Rb Hu, Ms, Rat 52922 node, using ab55152. MHC class I antibody [AF6-88.5.5.3] (Biotin) M Flow Cyt Ms Ms 93528 MHC Class II antibody MHC class I antibody [34-1-2S] (FITC) M Flow Cyt Ms Ms 95572 (ab55152) MHC class I antibody [34-1-2S] (Phycoerythrin) M Flow Cyt Ms Ms 95571 MHC Class I alpha antibody [F21-2] M AP, Flow Cyt, IP, WB Ms Chk 23483 Clonality Applications MHC Class 1 H2 Db antibody [27-11-13] - BSA and Azide free M Flow Cyt, FuncS, IHC-Fr Ms Ms 25244 M IHC-P, WB MHC Class 1 H2 Db antibody [28-14-8] (FITC) M Flow Cyt Ms Ms 25056 Host Species cross reactivity MHC Class 1 H2 Db antibody [KH95] M Flow Cyt, FuncS, WB Ms Ms 64373 Ms Hu, RecFrag MHC Class I H2 Dd antibody [34-2-12] M Flow Cyt, FuncS, IHC-Fr, IP Ms Ms 64368 MHC Class I H2 Dk antibody [15-5-5.3] M Flow Cyt Ms Ms 25216 MHC Class II antigens are a valuable tool for studying T helper cell interactions with class II MHC Class I H2 Kb antibody P ELISA, WB Rb Ms 93364 positive antigen presenting cells, as well as the MHC Class I H2 Kd + Dd + q + u + v antibody [1.B.552] (FITC) M Flow Cyt, IP Ms Ms 62228 development of T helper cells since the antigen is present on stromal cells in the thymus. -

PDIA3 Recombinant Protein Description Product Info
9853 Pacific Heights Blvd. Suite D. San Diego, CA 92121, USA Tel: 858-263-4982 Email: [email protected] 32-2652: PDIA3 Recombinant Protein ERp57,ERp60,ERp61,GRP57,GRP58,HsT17083,P58,PI-PLC,ER60,Protein disulfide-isomerase A3,Disulfide Alternative isomerase ER-60,Endoplasmic reticulum resident protein 60,ER protein 60,58 kDa microsomal Name : protein,Endoplasmic reticulum resident protein 57, Description Source : Escherichia Coli. PDIA3 Human Recombinant produced in E.Coli is a single, non-glycosylated polypeptide chain containing 518 amino acids (25-505 a.a.) and having a molecular wieght of 58.5 kDa. The PDIA3 is fused to 37 a.a. His-Tag at N-terminus and purified by proprietary chromatographic techniques. PDIA3 is an enzyme that belongs to the endoplasmic reticulum and interacts with lectin chaperones calreticulin and calnexin to modulate folding of newly synthesized glycoproteins. PDIA3 has protein disulfide isomerase activity. Complexes of lectins and PDIA3 mediate protein folding by promoting formation of disulfide bonds in their glycoprotein substrates. PDIA3 is expressed in the lumbar spinal cord from rats submitted to peripheral lesion during neonatal period. PDIA3 interacts with thiazide-sensitive sodium-chloride cotransporter in the kidney and is induced by glucose deprivation. PDIA3 is part of the major histocompatibility complex (MHC) class I peptide-loading complex (TAP1), which is important for formation of the final antigen conformation and export from the endoplasmic reticulum to the cell surface. Product Info Amount : 25 µg Purification : Greater than 95.0% as determined by SDS-PAGE. The PDIA3 protein solution contains 20mM Tris-HCl, pH-8, 1mM DTT, 0.1M NaCl and 10% Content : glycerol. -

Mechanisms of HIV Protein Degradation Into Epitopes: Implications for Vaccine Design
Viruses 2014, 6, 3271-3292; doi:10.3390/v6083271 OPEN ACCESS viruses ISSN 1999-4915 www.mdpi.com/journal/viruses Review Mechanisms of HIV Protein Degradation into Epitopes: Implications for Vaccine Design Marijana Rucevic †, Julie Boucau †, Jens Dinter †, Georgio Kourjian † and Sylvie Le Gall * Ragon Institute of MGH, MIT and Harvard, Massachusetts General Hospital and Harvard Medical School, Cambridge, MA 02139, USA; E-Mails: [email protected] (M.R.); [email protected] (J.B.); [email protected] (J.D.); [email protected] (G.K.) † These authors contributed equally to this work. * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +1-857-268-7010. Received: 11 June 2014; in revised form: 6 August 2014 / Accepted: 11 August 2014 / Published: 21 August 2014 Abstract: The degradation of HIV-derived proteins into epitopes displayed by MHC-I or MHC-II are the first events leading to the priming of HIV-specific immune responses and to the recognition of infected cells. Despite a wealth of information about peptidases involved in protein degradation, our knowledge of epitope presentation during HIV infection remains limited. Here we review current data on HIV protein degradation linking epitope production and immunodominance, viral evolution and impaired epitope presentation. We propose that an in-depth understanding of HIV antigen processing and presentation in relevant primary cells could be exploited to identify signatures leading to efficient or inefficient epitope presentation in HIV proteomes, and to improve the design of immunogens eliciting immune responses efficiently recognizing all infected cells. Keywords: HIV; antigen processing; protein degradation; proteasome; aminopeptidase; peptidase; immunogen; vaccine vector; dendritic cells; T cells; viral evolution Viruses 2014, 6 3272 1. -

Footprints of Antigen Processing Boost MHC Class II Natural Ligand
Barra et al. Genome Medicine (2018) 10:84 https://doi.org/10.1186/s13073-018-0594-6 RESEARCH Open Access Footprints of antigen processing boost MHC class II natural ligand predictions Carolina Barra1† , Bruno Alvarez1†, Sinu Paul2, Alessandro Sette2, Bjoern Peters2, Massimo Andreatta1, Søren Buus3 and Morten Nielsen1,4* Abstract Background: Major histocompatibility complex class II (MHC-II) molecules present peptide fragments to T cells for immune recognition. Current predictors for peptide to MHC-II binding are trained on binding affinity data, generated in vitro and therefore lacking information about antigen processing. Methods: We generate prediction models of peptide to MHC-II binding trained with naturally eluted ligands derived from mass spectrometry in addition to peptide binding affinity data sets. Results: We show that integrated prediction models incorporate identifiable rules of antigen processing. In fact, we observed detectable signals of protease cleavage at defined positions of the ligands. We also hypothesize a role of the length of the terminal ligand protrusions for trimming the peptide to the MHC presented ligand. Conclusions: The results of integrating binding affinity and eluted ligand data in a combined model demonstrate improved performance for the prediction of MHC-II ligands and T cell epitopes and foreshadow a new generation of improved peptide to MHC-II prediction tools accounting for the plurality of factors that determine natural presentation of antigens. Keywords: MHC-II, Binding predictions, Eluted ligands, T cell epitope, Neural networks, Antigen processing, Machine learning, Mass spectrometry Background MHC-II binding groove, unlike MHC class I, is open at Major histocompatibility complex class II (MHC-II) mole- both ends. -

Antigen Processing and Presentation Pamela Wearsch and Peter Cresswell
Antigen processing and presentation Pamela Wearsch and Peter Cresswell In antigen-presenting cells (APCs), such as dendritic cells (DCs) and B cells, heterogeneous intracellular phagocytosed by APCs, this simple division is not strictly enforced. Indeed, exogenous proteins pathways and mechanisms are responsible for generating complexes of MHC class I and class II internalized by DCs can generate peptide–MHC class I complexes that are recognized by CD8+ T cells, molecules with peptide antigens, and complexes of CD1 molecules with lipid antigens, for presentation a phenomenon referred to as cross-presentation. Similarly, endogenous and viral proteins can generate to T cells. This process — referred to as antigen processing and presentation — allows T cells to peptide–MHC class II complexes that are recognized by CD4+ T cells in a process involving autophagy. continuously assess the intracellular and extracellular milieu for signs of infection or abnormal cell Understanding the processes and mechanisms by which antigens are captured, processed and loaded growth. Although MHC class I molecules typically bind peptides derived from endogenous proteins onto MHC molecules for presentation to T cells provides us with crucial insights that are necessary for and MHC class II molecules typically bind peptides derived from proteins that are endocytosed or the design of vaccines and therapeutic strategies to bolster T-cell responses. The MHC class II pathway The MHC class I pathway MHC class II αβ-chain dimers are assembled in the endoplasmic reticulum (ER) All nucleated cells express MHC class I molecules and present endogenous peptide as a nonameric complex with the invariant chain (Ii), which protects against antigens to CD8+ T cells, but some DC subsets can also present exogenous premature peptide or protein interactions in pre-lysosomal peptides to CD8+ T cells through cross-presentation. -

Antigen Presentation to HLA Class II-Restricted Measles
Proc. Natl. Acad. Sci. USA Vol. 85, pp. 1209-1212, February 1988 Immunology Antigen presentation to HLA class II-restricted measles virus-specific T-cell clones can occur in the absence of the invariant chain (antigen processing/chloroquine/DNA-mediated gene transfer/HLA-DR restriction/major histocompatibility complex) RAFICK P. SEKALY*t, STEVEN JACOBSONt, JOHN R. RICHERT§, CECILE TONNELLE*¶, HENRY F. MCFARLANDt, AND ERIC 0. LONG* *Laboratory of Immunogenetics, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892; tNeuroimmunology Branch, National Institute of Neurological and Communicative Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892; and §Department of Neurology, Georgetown University Medical Center, Washington, DC 20007 Communicated by William E. Paul, November 9, 1987 ABSTRACT A human fibroblast line expressing HLA- The requirements for cell-surface expression of class II a/3 DR1 antigen on its surface was generated by transfection with heterodimers have also been analyzed by DNA-mediated DRa and DRP cDNAs. Expression of the invariant chain gene gene transfer. It was recently shown that the invariant chain was not detectable in the transfected fibroblasts and was not that is transiently associated with a,8 heterodimers intracel- induced by infection with measles virus. Lysis of measles lularly is not required for expression of human MHC class II virus-infected cells occurred with DRM- but not with DR4- antigens at the cell surface (8, 9). These results suggested restricted measles virus-specific cytotoxic T-lymphocyte (CTL) that the invariant chain might be involved in the function of clones and was inhibited by a monoclonal antibody specific for MHC class II antigens, such as binding of processed antigen DR antigen. -

MHC I Chaperone Complexes Shaping Immunity
Available online at www.sciencedirect.com ScienceDirect MHC I chaperone complexes shaping immunity Christoph Thomas and Robert Tampe´ Major histocompatibility complex class I (MHC I) molecules by displaying peptides at the cell surface on MHC I present peptides on the surface of most nucleated cells and heterodimers, which consist of the peptide-binding, poly- allow the immune system to detect and eliminate infected or morphic heavy chain, and the smaller b2-microglobulin malignantly transformed cells. The peptides are derived from (b2m). By scanning the pMHC I complexes via T-cell + endogenous proteins by proteasomal degradation or aberrant receptors (TCRs), antigen-experienced CD8 T lympho- translation, and are translocated from the cytosol into the cytes of the adaptive immune system are able to recognize endoplasmic reticulum (ER) by the transporter associated with foreign, antigenic peptides, and destroy infected or cancer- antigen processing (TAP), a central component of the peptide- ous cells [1,2]. The peptides presented by MHC I are loading complex (PLC). The peptides are subsequently predominantly a small subset of the protein fragments processed by ER-resident aminopeptidases (ERAP1/2) and generated by proteasomal degradation in the cytosol; they loaded onto MHC I. This loading, however, does not happen are selectively transported into the ER where theloading of indiscriminately: in a process called peptide editing or peptide the MHC I with peptidestakesplace. Peptidetranslocation proofreading, the MHC I-specific chaperones tapasin and into the ER is carried out by the heterodimeric ABC (ATP- TAPBPR (TAP-binding protein-related) catalyze the selection of binding cassette) transporter TAP [3]. Inside the ER, high-affinity peptides and stable peptide-MHC I (pMHC I) peptides can be further trimmed by the aminopeptidases complexes. -

Essential Glycan-Dependent Interactions Optimize MHC Class I Peptide Loading
Essential glycan-dependent interactions optimize MHC class I peptide loading Pamela A. Wearscha, David R. Peapera, and Peter Cresswella,b,1 aDepartment of Immunobiology and bDepartment of Cell Biology and Howard Hughes Medical Institute, Yale University School of Medicine, New Haven, CT 06520-8011 Contributed by Peter Cresswell, February 15, 2011 (sent for review January 5, 2011) In this study we sought to better understand the role of the (CNX). Both chaperones have a globular lectin-binding domain glycoprotein quality control machinery in the assembly of MHC and an extended arm known as the P-domain that binds specifi- class I molecules with high-affinity peptides. The lectin-like chap- cally to ERp57, a member of the protein disulfide isomerase (PDI) erone calreticulin (CRT) and the thiol oxidoreductase ERp57 partic- family. ERp57 has a four-domain architecture of abb′a′ in which fi ipate in the nal step of this process as part of the peptide-loading the first and last contain a CXXC active site. CRT and CNX work complex (PLC). We provide evidence for an MHC class I/CRT in- in concert with ERp57 to promote proper folding and disulfide termediate before PLC engagement and examine the nature of that bond formation of newly synthesized glycoproteins. Upon release chaperone interaction in detail. To investigate the mechanism of of the glycoprotein from CRT or CNX, its glycan is deglucosylated peptide loading and roles of individual components, we reconsti- tuted a PLC subcomplex, excluding the Transporter Associated with by GlsII and is no longer a substrate for the chaperones. If the Antigen Processing, from purified, recombinant proteins.