MHC Structure and Function − Antigen Presentation. Part 2 Estrutura Do MHC E Função − Apresentação De Antígenos
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
IFM Innate Immunity Infographic
UNDERSTANDING INNATE IMMUNITY INTRODUCTION The immune system is comprised of two arms that work together to protect the body – the innate and adaptive immune systems. INNATE ADAPTIVE γδ T Cell Dendritic B Cell Cell Macrophage Antibodies Natural Killer Lymphocites Neutrophil T Cell CD4+ CD8+ T Cell T Cell TIME 6 hours 12 hours 1 week INNATE IMMUNITY ADAPTIVE IMMUNITY Innate immunity is the body’s first The adaptive, or acquired, immune line of immunological response system is activated when the innate and reacts quickly to anything that immune system is not able to fully should not be present. address a threat, but responses are slow, taking up to a week to fully respond. Pathogen evades the innate Dendritic immune system T Cell Cell Through antigen Pathogen presentation, the dendritic cell informs T cells of the pathogen, which informs Macrophage B cells B Cell B cells create antibodies against the pathogen Macrophages engulf and destroy Antibodies label invading pathogens pathogens for destruction Scientists estimate innate immunity comprises approximately: The adaptive immune system develops of the immune memory of pathogen exposures, so that 80% system B and T cells can respond quickly to eliminate repeat invaders. IMMUNE SYSTEM AND DISEASE If the immune system consistently under-responds or over-responds, serious diseases can result. CANCER INFLAMMATION Innate system is TOO ACTIVE Innate system NOT ACTIVE ENOUGH Cancers grow and spread when tumor Certain diseases trigger the innate cells evade detection by the immune immune system to unnecessarily system. The innate immune system is respond and cause excessive inflammation. responsible for detecting cancer cells and This type of chronic inflammation is signaling to the adaptive immune system associated with autoimmune and for the destruction of the cancer cells. -

Antigen Presentation by Dendritic Cells and Their Significance in Antineoplastic Immunotherapy
in vivo 18: 81-100 (2004) Antigen Presentation by Dendritic Cells and their Significance in Antineoplastic Immunotherapy BELA BODEY1,2, STUART E. SIEGEL1,2 and HANS E. KAISER3 1Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA; 2Childrens’ Center for Cancer and Blood Diseases, Childrens’ Hospital Los Angeles, Los Angeles, CA; 3Department of Pathology, School of Medicine, University of Maryland, Baltimore, MD, U.S.A. and Department of General and Experimental Pathology, University of Vienna, Vienna, Austria Abstract. Dendritic cells (DCs) are present in essentially every derived peptides on the surface of major histocompatibility mammalian tissue, where they operate at the interface of innate complex (MHC) molecules and acquire the cellular specialization and acquired immunity by recognizing pathogens and presenting to select and activate naive antigen-specific T lymphocytes. pathogen-derived peptides to T lymphocytes. According to the Immunotherapeutic ideas are based on the ability of the research group of Shortman (1-9), experimental results suggest a mammalian immune system to recognize neoplastically "dual" DC differentiation model, demonstrating the existence of transformed cells. Immunotherapy of human neoplasms has both myeloid-derived (with characteristic IF: CD11b+, CD11c+, always represented a very attractive fourth-modality therapeutic CD8alpha- and DEC205+) and lymphoid-derived DCs (showing approach, especially in light of the many shortcomings of CD11b-, CD11c-, CD8alpha+ and DEC205+ IF). DCs, including conventional surgical, radiation and chemotherapies in the interdigitating cells (IDCs) and Langerhans cells (LCs), are management of neoplastically transformed cells. The cancer characterized by dendritic morphology, high migratory mobility and vaccine approach to therapy is based on the notion that the are the most effective, "professional" cells for antigen presentation immune system could possibly mount a rejection strength response in primary immune responses. -

Adoptive Cell Therapy Using Engineered Natural Killer Cells
Bone Marrow Transplantation (2019) 54:785–788 https://doi.org/10.1038/s41409-019-0601-6 REVIEW ARTICLE Adoptive cell therapy using engineered natural killer cells Katayoun Rezvani1 © The Author(s), under exclusive licence to Springer Nature Limited 2019 Abstract The generation of autologous T cells expressing a chimeric antigen receptor (CAR) have revolutionized the field of adoptive cellular therapy. CAR-T cells directed against CD19 have resulted in remarkable clinical responses in patients affected by B-lymphoid malignancies. However, the production of allogeneic CAR-T cells products remains expensive and clinically challenging. Moreover, the toxicity profile of CAR T-cells means that currently these life-saving treatments are only delivered in specialized centers. Therefore, efforts are underway to develop reliable off-the-shelf cellular products with acceptable safety profiles for the treatment of patients with cancer. Natural killer (NK) cells are innate effector lymphocytes with potent antitumor activity. The availability of NK cells from multiple sources and their proven safety profile in the allogeneic setting positions them as attractive contenders for cancer immunotherapy. In this review, we discuss advantages and potential drawbacks of using NK cells as a novel cellular therapy against hematologic malignancies, as well as strategies 1234567890();,: 1234567890();,: to further enhance their effector function. Introduction need for inpatient care may ultimately be economically unviable for many health care systems; (iii) the longer time Adoptive cell therapy has become a powerful treatment that is required to generate CAR T-cells may result in modality for advanced cancers refractory to conventional unavoidable delays in therapy, especially for patients with therapy. -

Antigen Processing (Major Hbitocompatlbility Complex/Class I Moecules/Lymphokines) YOUNG YANG, JAMES B
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 4928-4932, June 1992 Immunology Proteasomes are regulated by interferon y: Implications for antigen processing (major hbitocompatlbility complex/class I moecules/lymphokines) YOUNG YANG, JAMES B. WATERS, KLAUS FROH, AND PER A. PETERSON Department of Immunology, The Scripps Research Institute, La Jolla, CA 92037 Communicated by Frank J. Dixon, February 27, 1992 ABSTRACT Class I major histocompatibility complex strengthened by the findings, presented in this communica- (MHC) molecules present antigenic peptides of cytoplasmic tion, that several proteasomal subunits, including MHC- origin to T cells. As the lengths ofthese peptides seem stried encoded subunits, are regulated by interferon y (IFN--y) and to eight or nine amino acids, an unusual proteolytic system that the incorporation of several more subunits into protea- must play a role in antigen processing. Proteasomes, a major somes appears to depend on the expression of the MHC- extralysosomal proteolytic system, are responsible for the encoded proteasomal subunits. Moreover, the pattern of degradation of cytoplasmic proteins. We demonstrate that expression of IFN-y-regulated subunits suggests complexi- several proteasomal subunits, including MHC-encoded sub- ties in the regulation of proteasomes with respect to its units, are regulated by interferon y. These data and the finding subunit composition, subcellular localization, and its incor- that MHC-encoded and other interferon -regulated protea- poration into larger ubiquitin-related proteolytic complexes. somal subunits are uniquely associated with proteasomes Possible functions for the MHC-encoded and IFN-y- strongly suggest that the immune system has recruited protea- regulated proteasomal subunits in antigen processing are somes for antigen processing. -

Cytoplasmic Proteolysis Antigens on MHC Class II Is Dependent On
Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis This information is current as of September 28, 2021. Paushali Mukherjee, Aadish Dani, Sumeena Bhatia, Nagendra Singh, Alexander Y. Rudensky, Anna George, Vineeta Bal, Satyajit Mayor and Satyajit Rath J Immunol 2001; 167:2632-2641; ; doi: 10.4049/jimmunol.167.5.2632 Downloaded from http://www.jimmunol.org/content/167/5/2632 References This article cites 51 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/167/5/2632.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2001 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis1 Paushali Mukherjee,* Aadish Dani,† Sumeena Bhatia,* Nagendra Singh,* Alexander Y. -
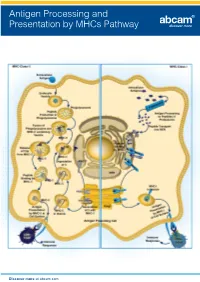
Antigen Processing and Presentation by Mhcs Pathway
Antigen Processing and Presentation by MHCs Pathway Discover more at abcam.com Antigen Processing and Presentation by MHCs Pathway Related antibodies Product This image shows MHC Class II highlights Product Clonality Applications Host Species Reactivity Product code expressing cells in formalin fixed and paraffin embedded human lymph MHC class I antibody [EP1395Y] M Flow Cyt, ICC/IF, IHC-P, IP, WB Rb Hu, Ms, Rat 52922 node, using ab55152. MHC class I antibody [AF6-88.5.5.3] (Biotin) M Flow Cyt Ms Ms 93528 MHC Class II antibody MHC class I antibody [34-1-2S] (FITC) M Flow Cyt Ms Ms 95572 (ab55152) MHC class I antibody [34-1-2S] (Phycoerythrin) M Flow Cyt Ms Ms 95571 MHC Class I alpha antibody [F21-2] M AP, Flow Cyt, IP, WB Ms Chk 23483 Clonality Applications MHC Class 1 H2 Db antibody [27-11-13] - BSA and Azide free M Flow Cyt, FuncS, IHC-Fr Ms Ms 25244 M IHC-P, WB MHC Class 1 H2 Db antibody [28-14-8] (FITC) M Flow Cyt Ms Ms 25056 Host Species cross reactivity MHC Class 1 H2 Db antibody [KH95] M Flow Cyt, FuncS, WB Ms Ms 64373 Ms Hu, RecFrag MHC Class I H2 Dd antibody [34-2-12] M Flow Cyt, FuncS, IHC-Fr, IP Ms Ms 64368 MHC Class I H2 Dk antibody [15-5-5.3] M Flow Cyt Ms Ms 25216 MHC Class II antigens are a valuable tool for studying T helper cell interactions with class II MHC Class I H2 Kb antibody P ELISA, WB Rb Ms 93364 positive antigen presenting cells, as well as the MHC Class I H2 Kd + Dd + q + u + v antibody [1.B.552] (FITC) M Flow Cyt, IP Ms Ms 62228 development of T helper cells since the antigen is present on stromal cells in the thymus. -

Natural Killer Cells Associated with SARS-Cov-2 Viral RNA Shedding
Bao et al. Exp Hematol Oncol (2021) 10:5 https://doi.org/10.1186/s40164-021-00199-1 Experimental Hematology & Oncology LETTER TO THE EDITOR Open Access Natural killer cells associated with SARS-CoV-2 viral RNA shedding, antibody response and mortality in COVID-19 patients Changqian Bao1†, Xiandong Tao2,3†, Wei Cui4†, Yuanyuan Hao1, Shuaike Zheng5, Bin Yi2,3, Tiewen Pan2, Ken H. Young6* and Wenbin Qian1,7* Abstract Coronavirus disease 2019 (COVID-19) is a novel infectious viral disease caused by the severe acute respiratory syn- drome coronavirus 2 (SARS-CoV-2). Two consecutively negative SARS-CoV-2 viral RNA test ( interval 24 hours), improved respiratory symptoms and obvious absorption of infammation in pulmonary imaging are≥ the discharge criteria for COVID-19 patients. The clearance profle of viral RNA in the upper respiratory tract specimens, including nasopharyngeal swab and/or oropharyngeal swabs, is related to innate immune cells such as Natural Killer cells. A total of 168 patients were included for the study. In this cohort, non-severe and severe groups showed signifcant dif- ferences in white blood cells, neutrophils, lymphocytes, basophils and platelets counts, as well as in infection related parameters such as CRP and serum cytokine IL-6. For lymphocyte subsets tests at admission, the severe group dis- played signifcantly lower cell counts than the non-severe group. Higher counts of total T cells, CD4 T cells, CD8 T cells, and NK cells in peripheral blood showed a signifcant correlation with the shorter time taken to+ obtain the frst+ negative viral RNA test and frst positive IgM/ IgG antibody test. -

Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases
antibodies Review Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases Courtney A. Iberg and Daniel Hawiger * Department of Molecular Microbiology and Immunology, Saint Louis University School of Medicine, Doisy Research Center, 1205 Carr Lane, St. Louis, MO 63104, USA; [email protected] * Correspondence: [email protected] Received: 31 March 2020; Accepted: 30 April 2020; Published: 15 June 2020 Abstract: The specific targeting of dendritic cells (DCs) using antigen-delivering antibodies has been established to be a highly efficient protocol for the induction of tolerance and protection from autoimmune processes in experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS), as well as in some other animal disease models. As the specific mechanisms of such induced tolerance are being investigated, the newly gained insights may also possibly help to design effective treatments for patients. Here we review approaches applied for the amelioration of autoimmunity in animal models based on antibody-mediated targeting of self-antigens to DCs. Further, we discuss relevant mechanisms of immunological tolerance that underlie such approaches, and we also offer some future perspectives for the application of similar methods in certain related disease settings such as transplantation. Keywords: dendritic cells; tolerance; antigen targeting; chimeric antibodies; autoimmunity; multiple sclerosis; diabetes 1. Introduction Over one hundred years ago, Paul Ehrlich coined the term “horror autotoxicus” to define an immune attack against an organism’s healthy tissues [1]. Since then, our knowledge of the complex mechanisms of the immune system as well as our understanding of the pathogenesis of specific autoimmune diseases have grown tremendously. -

Adaptive Immune Systems
Immunology 101 (for the Non-Immunologist) Abhinav Deol, MD Assistant Professor of Oncology Wayne State University/ Karmanos Cancer Institute, Detroit MI Presentation originally prepared and presented by Stephen Shiao MD, PhD Department of Radiation Oncology Cedars-Sinai Medical Center Disclosures Bristol-Myers Squibb – Contracted Research What is the immune system? A network of proteins, cells, tissues and organs all coordinated for one purpose: to defend one organism from another It is an infinitely adaptable system to combat the complex and endless variety of pathogens it must address Outline Structure of the immune system Anatomy of an immune response Role of the immune system in disease: infection, cancer and autoimmunity Organs of the Immune System Major organs of the immune system 1. Bone marrow – production of immune cells 2. Thymus – education of immune cells 3. Lymph Nodes – where an immune response is produced 4. Spleen – dual role for immune responses (especially antibody production) and cell recycling Origins of the Immune System B-Cell B-Cell Self-Renewing Common Progenitor Natural Killer Lymphoid Cell Progenitor Thymic T-Cell Selection Hematopoetic T-Cell Stem Cell Progenitor Dendritic Cell Myeloid Progenitor Granulocyte/M Macrophage onocyte Progenitor The Immune Response: The Art of War “Know your enemy and know yourself and you can fight a hundred battles without disaster.” -Sun Tzu, The Art of War Immunity: Two Systems and Their Key Players Adaptive Immunity Innate Immunity Dendritic cells (DC) B cells Phagocytes (Macrophages, Neutrophils) Natural Killer (NK) Cells T cells Dendritic Cells: “Commanders-in-Chief” • Function: Serve as the gateway between the innate and adaptive immune systems. -

Eosinophil Response Against Classical and Emerging
REVIEWS Eosinophil Response Against Classical and Emerging Respiratory Viruses: COVID-19 Rodrigo-Muñoz JM1,2, Sastre B1,2, Cañas JA1,2, Gil-Martínez M1, Redondo N1, del Pozo V1,2 1Immunology Department, Instituto de Investigación Sanitaria (IIS) Fundación Jiménez Díaz, Madrid, Spain 2CIBER de Enfermedades Respiratorias (CIBERES), Madrid, Spain J Investig Allergol Clin Immunol 2021; Vol. 31(2): 94-107 doi: 10.18176/jiaci.0624 Abstract Eosinophils were discovered more than 140 years ago. These polymorphonuclear leukocytes have a very active metabolism and contain numerous intracellular secretory granules that enable multiple effects on both health and disease status. Classically, eosinophils have been considered important immune cells in the pathogenesis of inflammatory processes (eg, parasitic helminth infections) and allergic or pulmonary diseases (eg, asthma) and are always associated with a type 2 immune response. Furthermore, in recent years, eosinophils have been linked to the immune response by conferring host protection against fungi, bacteria, and viruses, which they recognize through several molecules, such as toll-like receptors and the retinoic acid–inducible gene 1–like receptor. The immune protection provided by eosinophils is exerted through multiple mechanisms and properties. Eosinophils contain numerous cytoplasmatic granules that release cationic proteins, cytokines, chemokines, and other molecules, all of which contribute to their functioning. In addition to the competence of eosinophils as effector cells, their capabilities as antigen-presenting cells enable them to act in multiple situations, thus promoting diverse aspects of the immune response. This review summarizes various aspects of eosinophil biology, with emphasis on the mechanisms used and roles played by eosinophils in host defence against viral infections and response to vaccines. -

Understanding the Immune System: How It Works
Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute Understanding the Immune System How It Works U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NATIONAL INSTITUTES OF HEALTH National Institute of Allergy and Infectious Diseases National Cancer Institute NIH Publication No. 03-5423 September 2003 www.niaid.nih.gov www.nci.nih.gov Contents 1 Introduction 2 Self and Nonself 3 The Structure of the Immune System 7 Immune Cells and Their Products 19 Mounting an Immune Response 24 Immunity: Natural and Acquired 28 Disorders of the Immune System 34 Immunology and Transplants 36 Immunity and Cancer 39 The Immune System and the Nervous System 40 Frontiers in Immunology 45 Summary 47 Glossary Introduction he immune system is a network of Tcells, tissues*, and organs that work together to defend the body against attacks by “foreign” invaders. These are primarily microbes (germs)—tiny, infection-causing Bacteria: organisms such as bacteria, viruses, streptococci parasites, and fungi. Because the human body provides an ideal environment for many microbes, they try to break in. It is the immune system’s job to keep them out or, failing that, to seek out and destroy them. Virus: When the immune system hits the wrong herpes virus target or is crippled, however, it can unleash a torrent of diseases, including allergy, arthritis, or AIDS. The immune system is amazingly complex. It can recognize and remember millions of Parasite: different enemies, and it can produce schistosome secretions and cells to match up with and wipe out each one of them. -

Antibody-Antigen Interaction (Anti-Nucleotide Antibody/Anti-Hapten Antibody) ROBERT J
Proc. Nati. Acad. Sci. USA Vol. 77, No. 12, pp. 7410-7414, December 1980 Immunology Anti-AMP antibody precipitation of multiply adenylylated forms of glutamine synthetase from Escherichia coli: A model relating both concentration and density of antigenic sites with the antibody-antigen interaction (anti-nucleotide antibody/anti-hapten antibody) ROBERT J. HOHMAN*, SUE Goo RHEEt, AND EARL R. STADTMANtt tLaboratory of Biochemistry, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, Maryland 20205; and *Department of Microbiology, University of Maryland, College Park, Maryland 20742 Contributed by E. R. Stadtman, September 12, 1980 ABSTRACT Sheep antibodies directed against an AMP- ylylated forms of GS to determine the effects of the total con- bovine serum albumin conjugate exhibit highly specific binding centration of antigenic determinants, density of determinants toward AMP. These antibodies bind to the AMP moiety of ad- enylylated glutamine synthetase [-glutamate:ammonia ligase per antigen, and the topological distribution of antigenic de- (ADP-forming), EC 6.3.1.2] from Escherichia coli and to no other terminants, on the initial antibody-antigen binding, lattice antigenic determinant on the protein. E. coli glutamine syn- formation, and immunoprecipitation.§ Some of the experi- thetase can exist in variously modified (isomeric) forms that mental data were presented in an earlier preliminary com- differ with respect to the number (0-12) and the distribution of munication (3). identically adenylylated subunits [Ciardi, J. E., Cimino, F. & Stadtman, E. R. (1973) Biochemistry 12,432143301. Using this MATERIALS AND METHODS enzyme, together with the AMP-specific antibodies, we have investigated the effects of the total concentration, population Purification and Assay of GS.