Mechanisms of HIV Protein Degradation Into Epitopes: Implications for Vaccine Design
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Antigen Presentation by Dendritic Cells and Their Significance in Antineoplastic Immunotherapy
in vivo 18: 81-100 (2004) Antigen Presentation by Dendritic Cells and their Significance in Antineoplastic Immunotherapy BELA BODEY1,2, STUART E. SIEGEL1,2 and HANS E. KAISER3 1Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA; 2Childrens’ Center for Cancer and Blood Diseases, Childrens’ Hospital Los Angeles, Los Angeles, CA; 3Department of Pathology, School of Medicine, University of Maryland, Baltimore, MD, U.S.A. and Department of General and Experimental Pathology, University of Vienna, Vienna, Austria Abstract. Dendritic cells (DCs) are present in essentially every derived peptides on the surface of major histocompatibility mammalian tissue, where they operate at the interface of innate complex (MHC) molecules and acquire the cellular specialization and acquired immunity by recognizing pathogens and presenting to select and activate naive antigen-specific T lymphocytes. pathogen-derived peptides to T lymphocytes. According to the Immunotherapeutic ideas are based on the ability of the research group of Shortman (1-9), experimental results suggest a mammalian immune system to recognize neoplastically "dual" DC differentiation model, demonstrating the existence of transformed cells. Immunotherapy of human neoplasms has both myeloid-derived (with characteristic IF: CD11b+, CD11c+, always represented a very attractive fourth-modality therapeutic CD8alpha- and DEC205+) and lymphoid-derived DCs (showing approach, especially in light of the many shortcomings of CD11b-, CD11c-, CD8alpha+ and DEC205+ IF). DCs, including conventional surgical, radiation and chemotherapies in the interdigitating cells (IDCs) and Langerhans cells (LCs), are management of neoplastically transformed cells. The cancer characterized by dendritic morphology, high migratory mobility and vaccine approach to therapy is based on the notion that the are the most effective, "professional" cells for antigen presentation immune system could possibly mount a rejection strength response in primary immune responses. -

It Takes Two to Tango
HIGHLIGHTS IMMUNOTHERAPY It takes two to tango Much of the recent work on the development of active, specific immunotherapy of cancer has focused on the induction of CD8+ cytotoxic T-lymphocyte (CTL) responses, and several studies have shown that it is possible to stimulate tumour-specific CTLs using dendritic cells (DCs) that are loaded with tumour antigens. But CD4+ T-helper type 1 (TH1) cells are also important day of vaccination and loaded with various So, the results from this Phase I trial provide components of effective immune responses. MHC class-I- and -II-restricted peptides. convincing evidence that cryopreserved DCs In this study, Schuler–Thurner and Patients with incurable melanoma were can induce TH1 responses against tumour colleagues provide the first evidence that DCs treated with five biweekly vaccinations of antigens without significant toxicity, and it that are loaded with tumour-specific peptides DCs, followed by assessment one month also encourages further development of can rapidly induce TH1 responses in cancer after the final vaccination. DC-based vaccination technology. patients that are readily detectable ex vivo. The results showed that the vaccination Elaine Bell References and links The DCs that were used for vaccination protocol induced a rapid TH1 response in in this study were derived from blood patients, both to a control immunizing antigen ORIGINAL RESEARCH PAPER Schuler–Thurner, B. et al. Rapid induction of tumor-specific type 1 T helper cells in monocytes that were matured ex vivo using and also to defined MHC class-II-restricted metastatic melanoma patients by vaccination with mature, a defined cocktail (consisting of interleukin tumour antigens. -

Antigen Processing (Major Hbitocompatlbility Complex/Class I Moecules/Lymphokines) YOUNG YANG, JAMES B
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 4928-4932, June 1992 Immunology Proteasomes are regulated by interferon y: Implications for antigen processing (major hbitocompatlbility complex/class I moecules/lymphokines) YOUNG YANG, JAMES B. WATERS, KLAUS FROH, AND PER A. PETERSON Department of Immunology, The Scripps Research Institute, La Jolla, CA 92037 Communicated by Frank J. Dixon, February 27, 1992 ABSTRACT Class I major histocompatibility complex strengthened by the findings, presented in this communica- (MHC) molecules present antigenic peptides of cytoplasmic tion, that several proteasomal subunits, including MHC- origin to T cells. As the lengths ofthese peptides seem stried encoded subunits, are regulated by interferon y (IFN--y) and to eight or nine amino acids, an unusual proteolytic system that the incorporation of several more subunits into protea- must play a role in antigen processing. Proteasomes, a major somes appears to depend on the expression of the MHC- extralysosomal proteolytic system, are responsible for the encoded proteasomal subunits. Moreover, the pattern of degradation of cytoplasmic proteins. We demonstrate that expression of IFN-y-regulated subunits suggests complexi- several proteasomal subunits, including MHC-encoded sub- ties in the regulation of proteasomes with respect to its units, are regulated by interferon y. These data and the finding subunit composition, subcellular localization, and its incor- that MHC-encoded and other interferon -regulated protea- poration into larger ubiquitin-related proteolytic complexes. somal subunits are uniquely associated with proteasomes Possible functions for the MHC-encoded and IFN-y- strongly suggest that the immune system has recruited protea- regulated proteasomal subunits in antigen processing are somes for antigen processing. -

Cytoplasmic Proteolysis Antigens on MHC Class II Is Dependent On
Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis This information is current as of September 28, 2021. Paushali Mukherjee, Aadish Dani, Sumeena Bhatia, Nagendra Singh, Alexander Y. Rudensky, Anna George, Vineeta Bal, Satyajit Mayor and Satyajit Rath J Immunol 2001; 167:2632-2641; ; doi: 10.4049/jimmunol.167.5.2632 Downloaded from http://www.jimmunol.org/content/167/5/2632 References This article cites 51 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/167/5/2632.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2001 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis1 Paushali Mukherjee,* Aadish Dani,† Sumeena Bhatia,* Nagendra Singh,* Alexander Y. -

1 ICAM3-Fc Outperforms Receptor-Specific Antibodies
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 10 April 2019 doi:10.20944/preprints201904.0118.v1 Peer-reviewed version available at Molecules 2019, 24, 1825; doi:10.3390/molecules24091825 ICAM3-Fc outperforms receptor-specific antibodies targeted nanoparticles to dendritic cells for cross-presentation Luis J. Cruz,1* Paul J. Tacken,2 Johan S. van der Schoot,2 Felix Rueda,3 Ruurd Torensma,2 Carl G. Figdor2* 1Translational Nanobiomaterials and Imaging, Department of Radiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands. 2Department of Tumor Immunology, Radboud Insititute for Molecular Life Sciences, Radboud University Medical Center, Postbox 9101, 6500 HB Nijmegen, The Netherlands. 3Department of Biochemistry and Molecular Biology, University of Barcelona, Diagonal 643, 08028 Barcelona, Spain. Running title: ICAM3-Fc- versus antibody-targeted NP vaccines to human DCs Keywords: Delivery system; nanoparticle; targeting. AUTHOR INFORMATION Corresponding Author *Dr. Luis J. Cruz Translational Nanobiomaterials and Imaging, Department of Radiology, Bldg.1, C5-60. Leiden University Medical Center, Albinusdreef 2 2333 ZA Leiden, The Netherlands Tel: +31 71 5263025 Email: [email protected] *Prof. Dr. Carl G. Figdor Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, Postbox 9101, 6500 HB Nijmegen, The Netherlands. Fax: +31-24-3540339; Tel.: +31-24-3617600; E-mail: [email protected] 1 © 2019 by the author(s). Distributed -

Regulation of Calreticulin–Major Histocompatibility Complex (MHC) Class I Interactions by ATP
Regulation of calreticulin–major histocompatibility complex (MHC) class I interactions by ATP Sanjeeva Joseph Wijeyesakerea, Jessica K. Gagnonb, Karunesh Arorab, Charles L. Brooks IIIb, and Malini Raghavana,1 aDepartment of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, MI 48109; and bDepartment of Chemistry, University of Michigan, Ann Arbor, MI 48109 Edited by Peter Cresswell, Yale University School of Medicine, New Haven, CT, and approved September 1, 2015 (received for review May 26, 2015) The MHC class I peptide loading complex (PLC) facilitates the assembly computational methods validated by experimental approaches, we of MHC class I molecules with peptides, but factors that regulate the identify residues within the globular domain of calreticulin that stability and dynamics of the assembly complex are largely unchar- are important for ATP binding and ATPase activity. Based on acterized. Based on initial findings that ATP, in addition to MHC class further investigations into the functional effects of calreticulin I-specific peptide, is able to induce MHC class I dissociation from the mutants with deficiencies in ATP interactions, we elucidate a key PLC, we investigated the interaction of ATP with the chaperone role for ATP in the regulation of PLC dynamics and the in- calreticulin, an endoplasmic reticulum (ER) luminal, calcium-bind- teraction of calreticulin with other cellular proteins. ing component of the PLC that is known to bind ATP. We combined computational and experimental measurements to identify residues Results within the globular domain of calreticulin, in proximity to the high- ATP Destabilizes the PLC, Promoting MHC Class I Release. Calreticulin- affinity calcium-binding site, that are important for high-affinity ATP deficient mouse embryonic fibroblasts (MEFs) reconstituted binding and for ATPase activity. -
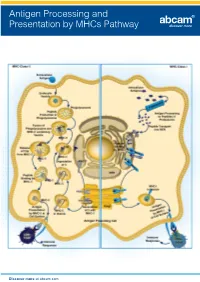
Antigen Processing and Presentation by Mhcs Pathway
Antigen Processing and Presentation by MHCs Pathway Discover more at abcam.com Antigen Processing and Presentation by MHCs Pathway Related antibodies Product This image shows MHC Class II highlights Product Clonality Applications Host Species Reactivity Product code expressing cells in formalin fixed and paraffin embedded human lymph MHC class I antibody [EP1395Y] M Flow Cyt, ICC/IF, IHC-P, IP, WB Rb Hu, Ms, Rat 52922 node, using ab55152. MHC class I antibody [AF6-88.5.5.3] (Biotin) M Flow Cyt Ms Ms 93528 MHC Class II antibody MHC class I antibody [34-1-2S] (FITC) M Flow Cyt Ms Ms 95572 (ab55152) MHC class I antibody [34-1-2S] (Phycoerythrin) M Flow Cyt Ms Ms 95571 MHC Class I alpha antibody [F21-2] M AP, Flow Cyt, IP, WB Ms Chk 23483 Clonality Applications MHC Class 1 H2 Db antibody [27-11-13] - BSA and Azide free M Flow Cyt, FuncS, IHC-Fr Ms Ms 25244 M IHC-P, WB MHC Class 1 H2 Db antibody [28-14-8] (FITC) M Flow Cyt Ms Ms 25056 Host Species cross reactivity MHC Class 1 H2 Db antibody [KH95] M Flow Cyt, FuncS, WB Ms Ms 64373 Ms Hu, RecFrag MHC Class I H2 Dd antibody [34-2-12] M Flow Cyt, FuncS, IHC-Fr, IP Ms Ms 64368 MHC Class I H2 Dk antibody [15-5-5.3] M Flow Cyt Ms Ms 25216 MHC Class II antigens are a valuable tool for studying T helper cell interactions with class II MHC Class I H2 Kb antibody P ELISA, WB Rb Ms 93364 positive antigen presenting cells, as well as the MHC Class I H2 Kd + Dd + q + u + v antibody [1.B.552] (FITC) M Flow Cyt, IP Ms Ms 62228 development of T helper cells since the antigen is present on stromal cells in the thymus. -

Efficient Elimination of Primary B-ALL Cells in Vitro and in Vivo Using A
Open access Original research J Immunother Cancer: first published as 10.1136/jitc-2020-000896 on 11 August 2020. Downloaded from Efficient elimination of primary B- ALL cells in vitro and in vivo using a novel 4- 1BB- based CAR targeting a membrane- distal CD22 epitope 1 1 1 Talia Velasco- Hernandez , Samanta Romina Zanetti , Heleia Roca- Ho, Francisco Gutierrez- Aguera,1 Paolo Petazzi,1 Diego Sánchez- Martínez,1 Oscar Molina,1 Matteo Libero Baroni,1 Jose Luis Fuster,2 Paola Ballerini,3 1,4 5 6,7 Clara Bueno, Narcis Fernandez- Fuentes, Pablo Engel , Pablo Menendez1,4,7,8 To cite: Velasco- Hernandez T, ABSTRACT BACKGROUND Zanetti SR, Roca- Ho H, et al. Background There are few therapeutic options available for B-cell acute lymphoblastic leukemia (B-ALL) Efficient elimination of primary patients with B- cell acute lymphoblastic leukemia (B- ALL) is an aggressive cancer, diagnosed at any age B- ALL cells in vitro and in vivo – using a novel 4- 1BB- based relapsing as CD19 either after chemotherapy or CD19- throughout the lifespan of an individual, CAR targeting a membrane- targeted immunotherapies. CD22- chimeric antigen receptor being the most common malignancy in distal CD22 epitope. Journal (CAR) T cells represent an attractive addition to CD19- CAR children.1 Despite current 5-year disease- + – for ImmunoTherapy of Cancer T cell therapy because they will target both CD22 CD19 free survival rates of ~80%, refractory and 2020;8:e000896. doi:10.1136/ B- ALL relapses and CD19– preleukemic cells. However, jitc-2020-000896 relapsed (R/R) patients have a dismal prog- the immune escape mechanisms from CD22- CAR T cells, nosis.2–7 Adult B- ALL is less frequent, but is and the potential contribution of the epitope binding of the commonly associated with an unfavorable ► Additional material is anti-CD22 single-chain variable fragment (scFv) remain published online only. -

Cell Biology from an Immune Perspective in This Lecture We Will
Harvard-MIT Division of Health Sciences and Technology HST.176: Cellular and Molecular Immunology Course Director: Dr. Shiv Pillai Cell Biology from an Immune Perspective In this lecture we will very briefly review some aspects of cell biology which are required as background knowledge in order to understand how the immune system works. These will include: 1. A brief overview of protein trafficking 2. Signal transduction 3. The cell cycle Some of these issues will be treated in greater depth in later lectures. Protein Trafficking/The Secretory Pathway: From an immune perspective the secretory compartment and structures enclosed by vesicles are “seen” in different ways from proteins that reside in the cytosol or the nucleus. We will briefly review the secretory and endocytic pathways and discuss the biogenesis of membrane proteins. Some of the issues that will be discussed are summarized in Figures 1-3. Early endosomes Late endosomes Lysosomes Golgi Vesiculo-Tubular Compartment ER Figure 1. An overview of the secretory pathway Early endosomes Late endosomes Multivesicular and multilamellar bodies Golgi ER Proteasomes Figure 2. Protein degradation occurs mainly in lysosomes and proteasomes Proteins that enter the cell from the environment are primarily degraded in lysosomes. Most cytosolic and nuclear proteins are degraded in organelles called proteasomes. Intriguingly these two sites of degradation are each functionally linked to distinct antigen presentation pathways, different kinds of MHC molecules and the activation of different categories of T cells. Integral membrane proteins maybe inserted into the membrane in a number of ways, the two most common of these ways being considered in Figure 3. -

Innate Immunity: MHC Class I As a Negative Regulator of TLR Signalling
RESEARCH HIGHLIGHTS IN BRIEF T RANSPLANTATION Handling complement for transplant success Haematopoietic cell transplantation (HCT) is used to treat cancer and blood disorders, but a potentially lethal complication is the development of graft-versus-host disease (GVHD). Total-body irradiation (TBI) is required to facilitate HCT and can promote GVHD by activating host dendritic cells (DCs), although the mechanisms involved are not completely understood. This study shows that, in mice, TBI causes host DCs to upregulate the complement components C3, C5a, factor B and factor D, and to downregulate CD55, a negative regulator of the complement cascade. In HCT using CD55-deficient donor T cells, recipient mice developed exacerbated GVHD, suggesting that upregulation of complement components by host DCs promotes the activation of allogeneic donor T cells. Indeed, less severe GVHD was seen in HCT using donor T cells that were deficient in the C3a and C5a receptors. Treatment of recipients with a C5a receptor antagonist during the post-transplantation period reduced GVHD development, and could be a useful strategy for preventing GVHD in humans after transplantation. ORIGINAL RESEARCH PAPER Kwan, W.-H. et al. Antigen-presenting cell-derived complement modulates graft-versus-host disease. J. Clin. Invest. 15 May 2012 (doi:10.1172/JCI61019) T CELLS ‘Leaky’ cytokine secretion by immunological synapses This study used live-cell imaging to investigate whether cytokine secretion by activated T cells is restricted by the immunological synapse to antigen-specific target cells or also affects bystander cells in the local environment. Responses to interferon-γ (IFNγ) were monitored by tracking the intracellular localization of a fluorescent STAT1 protein in ovalbumin- expressing and non-expressing astrocytes cultured with activated ovalbumin-specific CD8+ T cells. -

Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation
University of Massachusetts Medical School eScholarship@UMMS Open Access Publications by UMMS Authors 2021-03-09 Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation Karthik Dhatchinamoorthy University of Massachusetts Medical School Et al. Let us know how access to this document benefits ou.y Follow this and additional works at: https://escholarship.umassmed.edu/oapubs Part of the Amino Acids, Peptides, and Proteins Commons, Biological Factors Commons, Cancer Biology Commons, Hemic and Immune Systems Commons, Immunopathology Commons, Neoplasms Commons, and the Pathology Commons Repository Citation Dhatchinamoorthy K, Colbert JD, Rock KL. (2021). Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation. Open Access Publications by UMMS Authors. https://doi.org/10.3389/ fimmu.2021.636568. Retrieved from https://escholarship.umassmed.edu/oapubs/4636 Creative Commons License This work is licensed under a Creative Commons Attribution 4.0 License. This material is brought to you by eScholarship@UMMS. It has been accepted for inclusion in Open Access Publications by UMMS Authors by an authorized administrator of eScholarship@UMMS. For more information, please contact [email protected]. REVIEW published: 09 March 2021 doi: 10.3389/fimmu.2021.636568 Cancer Immune Evasion Through Loss of MHC Class I Antigen Presentation Karthik Dhatchinamoorthy, Jeff D. Colbert and Kenneth L. Rock* Department of Pathology, UMass Medical School, Worcester, MA, United States Major histocompatibility class I (MHC I) molecules bind peptides derived from a cell’s expressed genes and then transport and display this antigenic information on the cell surface. This allows CD8T cells to identify pathological cells that are synthesizing abnormal proteins, such as cancers that are expressing mutated proteins. -

The MHC Class I–Like Fc Receptor Promotes Humorally Mediated Autoimmune Disease Shreeram Akilesh, Stefka Petkova, Thomas J
Research article The MHC class I–like Fc receptor promotes humorally mediated autoimmune disease Shreeram Akilesh, Stefka Petkova, Thomas J. Sproule, Daniel J. Shaffer, Gregory J. Christianson, and Derry Roopenian The Jackson Laboratory, Bar Harbor, Maine, USA. The MHC class I family–like Fc receptor, FcRn, is normally responsible for extending the life span of serum IgG Ab’s, but whether this molecule contributes to autoimmune pathogenesis remains speculative. To deter- mine directly whether this function contributes to humoral autoimmune disease, we examined whether a defi- ciency in the FcRn heavy chain influences autoimmune arthritis in the K/BxN mouse model. FcRn deficiency conferred either partial or complete protection in the arthritogenic serum transfer and the more aggressive genetically determined K/BxN autoimmune arthritis models. The protective effects of an FcRn deficiency could be overridden with excessive amounts of pathogenic IgG Ab’s. The therapeutic saturation of FcRn by high-dose intravenous IgG (IVIg) also ameliorated arthritis, directly implicating FcRn blockade as a signifi- cant mechanism of IVIg’s anti-inflammatory action. The results suggest that FcRn is a potential therapeutic target that links the initiation and effector phases of humoral autoimmune disease. Introduction mice been used as a model for addressing this question with mixed At the inductive phase of a humoral autoimmune response, B results (refs. 9–16; D. Roopenian, unpublished observations). This cells, after encounter with APCs and T cells, undergo antigen- is not surprising because β2m controls many immunological and driven proliferation and differentiation into Ab-secreting plasma nonimmunological processes, including the development and cells. During the effector phase, Ab’s bind autoantigen leading function of CD8 T cells, natural T cells, conventional NK cells (17), to downstream events such as activation of complement, recruit- and iron homeostasis (18).