Major Histocompatibility Antigens and Antigen-Processing Molecules in Uveal Melanoma1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
IFM Innate Immunity Infographic
UNDERSTANDING INNATE IMMUNITY INTRODUCTION The immune system is comprised of two arms that work together to protect the body – the innate and adaptive immune systems. INNATE ADAPTIVE γδ T Cell Dendritic B Cell Cell Macrophage Antibodies Natural Killer Lymphocites Neutrophil T Cell CD4+ CD8+ T Cell T Cell TIME 6 hours 12 hours 1 week INNATE IMMUNITY ADAPTIVE IMMUNITY Innate immunity is the body’s first The adaptive, or acquired, immune line of immunological response system is activated when the innate and reacts quickly to anything that immune system is not able to fully should not be present. address a threat, but responses are slow, taking up to a week to fully respond. Pathogen evades the innate Dendritic immune system T Cell Cell Through antigen Pathogen presentation, the dendritic cell informs T cells of the pathogen, which informs Macrophage B cells B Cell B cells create antibodies against the pathogen Macrophages engulf and destroy Antibodies label invading pathogens pathogens for destruction Scientists estimate innate immunity comprises approximately: The adaptive immune system develops of the immune memory of pathogen exposures, so that 80% system B and T cells can respond quickly to eliminate repeat invaders. IMMUNE SYSTEM AND DISEASE If the immune system consistently under-responds or over-responds, serious diseases can result. CANCER INFLAMMATION Innate system is TOO ACTIVE Innate system NOT ACTIVE ENOUGH Cancers grow and spread when tumor Certain diseases trigger the innate cells evade detection by the immune immune system to unnecessarily system. The innate immune system is respond and cause excessive inflammation. responsible for detecting cancer cells and This type of chronic inflammation is signaling to the adaptive immune system associated with autoimmune and for the destruction of the cancer cells. -

Antigen Presentation by Dendritic Cells and Their Significance in Antineoplastic Immunotherapy
in vivo 18: 81-100 (2004) Antigen Presentation by Dendritic Cells and their Significance in Antineoplastic Immunotherapy BELA BODEY1,2, STUART E. SIEGEL1,2 and HANS E. KAISER3 1Department of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA; 2Childrens’ Center for Cancer and Blood Diseases, Childrens’ Hospital Los Angeles, Los Angeles, CA; 3Department of Pathology, School of Medicine, University of Maryland, Baltimore, MD, U.S.A. and Department of General and Experimental Pathology, University of Vienna, Vienna, Austria Abstract. Dendritic cells (DCs) are present in essentially every derived peptides on the surface of major histocompatibility mammalian tissue, where they operate at the interface of innate complex (MHC) molecules and acquire the cellular specialization and acquired immunity by recognizing pathogens and presenting to select and activate naive antigen-specific T lymphocytes. pathogen-derived peptides to T lymphocytes. According to the Immunotherapeutic ideas are based on the ability of the research group of Shortman (1-9), experimental results suggest a mammalian immune system to recognize neoplastically "dual" DC differentiation model, demonstrating the existence of transformed cells. Immunotherapy of human neoplasms has both myeloid-derived (with characteristic IF: CD11b+, CD11c+, always represented a very attractive fourth-modality therapeutic CD8alpha- and DEC205+) and lymphoid-derived DCs (showing approach, especially in light of the many shortcomings of CD11b-, CD11c-, CD8alpha+ and DEC205+ IF). DCs, including conventional surgical, radiation and chemotherapies in the interdigitating cells (IDCs) and Langerhans cells (LCs), are management of neoplastically transformed cells. The cancer characterized by dendritic morphology, high migratory mobility and vaccine approach to therapy is based on the notion that the are the most effective, "professional" cells for antigen presentation immune system could possibly mount a rejection strength response in primary immune responses. -

It Takes Two to Tango
HIGHLIGHTS IMMUNOTHERAPY It takes two to tango Much of the recent work on the development of active, specific immunotherapy of cancer has focused on the induction of CD8+ cytotoxic T-lymphocyte (CTL) responses, and several studies have shown that it is possible to stimulate tumour-specific CTLs using dendritic cells (DCs) that are loaded with tumour antigens. But CD4+ T-helper type 1 (TH1) cells are also important day of vaccination and loaded with various So, the results from this Phase I trial provide components of effective immune responses. MHC class-I- and -II-restricted peptides. convincing evidence that cryopreserved DCs In this study, Schuler–Thurner and Patients with incurable melanoma were can induce TH1 responses against tumour colleagues provide the first evidence that DCs treated with five biweekly vaccinations of antigens without significant toxicity, and it that are loaded with tumour-specific peptides DCs, followed by assessment one month also encourages further development of can rapidly induce TH1 responses in cancer after the final vaccination. DC-based vaccination technology. patients that are readily detectable ex vivo. The results showed that the vaccination Elaine Bell References and links The DCs that were used for vaccination protocol induced a rapid TH1 response in in this study were derived from blood patients, both to a control immunizing antigen ORIGINAL RESEARCH PAPER Schuler–Thurner, B. et al. Rapid induction of tumor-specific type 1 T helper cells in monocytes that were matured ex vivo using and also to defined MHC class-II-restricted metastatic melanoma patients by vaccination with mature, a defined cocktail (consisting of interleukin tumour antigens. -

Adoptive Cell Therapy Using Engineered Natural Killer Cells
Bone Marrow Transplantation (2019) 54:785–788 https://doi.org/10.1038/s41409-019-0601-6 REVIEW ARTICLE Adoptive cell therapy using engineered natural killer cells Katayoun Rezvani1 © The Author(s), under exclusive licence to Springer Nature Limited 2019 Abstract The generation of autologous T cells expressing a chimeric antigen receptor (CAR) have revolutionized the field of adoptive cellular therapy. CAR-T cells directed against CD19 have resulted in remarkable clinical responses in patients affected by B-lymphoid malignancies. However, the production of allogeneic CAR-T cells products remains expensive and clinically challenging. Moreover, the toxicity profile of CAR T-cells means that currently these life-saving treatments are only delivered in specialized centers. Therefore, efforts are underway to develop reliable off-the-shelf cellular products with acceptable safety profiles for the treatment of patients with cancer. Natural killer (NK) cells are innate effector lymphocytes with potent antitumor activity. The availability of NK cells from multiple sources and their proven safety profile in the allogeneic setting positions them as attractive contenders for cancer immunotherapy. In this review, we discuss advantages and potential drawbacks of using NK cells as a novel cellular therapy against hematologic malignancies, as well as strategies 1234567890();,: 1234567890();,: to further enhance their effector function. Introduction need for inpatient care may ultimately be economically unviable for many health care systems; (iii) the longer time Adoptive cell therapy has become a powerful treatment that is required to generate CAR T-cells may result in modality for advanced cancers refractory to conventional unavoidable delays in therapy, especially for patients with therapy. -

Antigen Processing (Major Hbitocompatlbility Complex/Class I Moecules/Lymphokines) YOUNG YANG, JAMES B
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 4928-4932, June 1992 Immunology Proteasomes are regulated by interferon y: Implications for antigen processing (major hbitocompatlbility complex/class I moecules/lymphokines) YOUNG YANG, JAMES B. WATERS, KLAUS FROH, AND PER A. PETERSON Department of Immunology, The Scripps Research Institute, La Jolla, CA 92037 Communicated by Frank J. Dixon, February 27, 1992 ABSTRACT Class I major histocompatibility complex strengthened by the findings, presented in this communica- (MHC) molecules present antigenic peptides of cytoplasmic tion, that several proteasomal subunits, including MHC- origin to T cells. As the lengths ofthese peptides seem stried encoded subunits, are regulated by interferon y (IFN--y) and to eight or nine amino acids, an unusual proteolytic system that the incorporation of several more subunits into protea- must play a role in antigen processing. Proteasomes, a major somes appears to depend on the expression of the MHC- extralysosomal proteolytic system, are responsible for the encoded proteasomal subunits. Moreover, the pattern of degradation of cytoplasmic proteins. We demonstrate that expression of IFN-y-regulated subunits suggests complexi- several proteasomal subunits, including MHC-encoded sub- ties in the regulation of proteasomes with respect to its units, are regulated by interferon y. These data and the finding subunit composition, subcellular localization, and its incor- that MHC-encoded and other interferon -regulated protea- poration into larger ubiquitin-related proteolytic complexes. somal subunits are uniquely associated with proteasomes Possible functions for the MHC-encoded and IFN-y- strongly suggest that the immune system has recruited protea- regulated proteasomal subunits in antigen processing are somes for antigen processing. -

Cytoplasmic Proteolysis Antigens on MHC Class II Is Dependent On
Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis This information is current as of September 28, 2021. Paushali Mukherjee, Aadish Dani, Sumeena Bhatia, Nagendra Singh, Alexander Y. Rudensky, Anna George, Vineeta Bal, Satyajit Mayor and Satyajit Rath J Immunol 2001; 167:2632-2641; ; doi: 10.4049/jimmunol.167.5.2632 Downloaded from http://www.jimmunol.org/content/167/5/2632 References This article cites 51 articles, 20 of which you can access for free at: http://www.jimmunol.org/content/167/5/2632.full#ref-list-1 http://www.jimmunol.org/ Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 28, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2001 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Efficient Presentation of Both Cytosolic and Endogenous Transmembrane Protein Antigens on MHC Class II Is Dependent on Cytoplasmic Proteolysis1 Paushali Mukherjee,* Aadish Dani,† Sumeena Bhatia,* Nagendra Singh,* Alexander Y. -

1 ICAM3-Fc Outperforms Receptor-Specific Antibodies
Preprints (www.preprints.org) | NOT PEER-REVIEWED | Posted: 10 April 2019 doi:10.20944/preprints201904.0118.v1 Peer-reviewed version available at Molecules 2019, 24, 1825; doi:10.3390/molecules24091825 ICAM3-Fc outperforms receptor-specific antibodies targeted nanoparticles to dendritic cells for cross-presentation Luis J. Cruz,1* Paul J. Tacken,2 Johan S. van der Schoot,2 Felix Rueda,3 Ruurd Torensma,2 Carl G. Figdor2* 1Translational Nanobiomaterials and Imaging, Department of Radiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands. 2Department of Tumor Immunology, Radboud Insititute for Molecular Life Sciences, Radboud University Medical Center, Postbox 9101, 6500 HB Nijmegen, The Netherlands. 3Department of Biochemistry and Molecular Biology, University of Barcelona, Diagonal 643, 08028 Barcelona, Spain. Running title: ICAM3-Fc- versus antibody-targeted NP vaccines to human DCs Keywords: Delivery system; nanoparticle; targeting. AUTHOR INFORMATION Corresponding Author *Dr. Luis J. Cruz Translational Nanobiomaterials and Imaging, Department of Radiology, Bldg.1, C5-60. Leiden University Medical Center, Albinusdreef 2 2333 ZA Leiden, The Netherlands Tel: +31 71 5263025 Email: [email protected] *Prof. Dr. Carl G. Figdor Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences, Radboud University Nijmegen Medical Centre, Postbox 9101, 6500 HB Nijmegen, The Netherlands. Fax: +31-24-3540339; Tel.: +31-24-3617600; E-mail: [email protected] 1 © 2019 by the author(s). Distributed -

Regulation of Calreticulin–Major Histocompatibility Complex (MHC) Class I Interactions by ATP
Regulation of calreticulin–major histocompatibility complex (MHC) class I interactions by ATP Sanjeeva Joseph Wijeyesakerea, Jessica K. Gagnonb, Karunesh Arorab, Charles L. Brooks IIIb, and Malini Raghavana,1 aDepartment of Microbiology and Immunology, University of Michigan Medical School, Ann Arbor, MI 48109; and bDepartment of Chemistry, University of Michigan, Ann Arbor, MI 48109 Edited by Peter Cresswell, Yale University School of Medicine, New Haven, CT, and approved September 1, 2015 (received for review May 26, 2015) The MHC class I peptide loading complex (PLC) facilitates the assembly computational methods validated by experimental approaches, we of MHC class I molecules with peptides, but factors that regulate the identify residues within the globular domain of calreticulin that stability and dynamics of the assembly complex are largely unchar- are important for ATP binding and ATPase activity. Based on acterized. Based on initial findings that ATP, in addition to MHC class further investigations into the functional effects of calreticulin I-specific peptide, is able to induce MHC class I dissociation from the mutants with deficiencies in ATP interactions, we elucidate a key PLC, we investigated the interaction of ATP with the chaperone role for ATP in the regulation of PLC dynamics and the in- calreticulin, an endoplasmic reticulum (ER) luminal, calcium-bind- teraction of calreticulin with other cellular proteins. ing component of the PLC that is known to bind ATP. We combined computational and experimental measurements to identify residues Results within the globular domain of calreticulin, in proximity to the high- ATP Destabilizes the PLC, Promoting MHC Class I Release. Calreticulin- affinity calcium-binding site, that are important for high-affinity ATP deficient mouse embryonic fibroblasts (MEFs) reconstituted binding and for ATPase activity. -
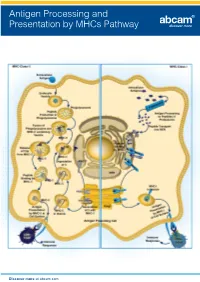
Antigen Processing and Presentation by Mhcs Pathway
Antigen Processing and Presentation by MHCs Pathway Discover more at abcam.com Antigen Processing and Presentation by MHCs Pathway Related antibodies Product This image shows MHC Class II highlights Product Clonality Applications Host Species Reactivity Product code expressing cells in formalin fixed and paraffin embedded human lymph MHC class I antibody [EP1395Y] M Flow Cyt, ICC/IF, IHC-P, IP, WB Rb Hu, Ms, Rat 52922 node, using ab55152. MHC class I antibody [AF6-88.5.5.3] (Biotin) M Flow Cyt Ms Ms 93528 MHC Class II antibody MHC class I antibody [34-1-2S] (FITC) M Flow Cyt Ms Ms 95572 (ab55152) MHC class I antibody [34-1-2S] (Phycoerythrin) M Flow Cyt Ms Ms 95571 MHC Class I alpha antibody [F21-2] M AP, Flow Cyt, IP, WB Ms Chk 23483 Clonality Applications MHC Class 1 H2 Db antibody [27-11-13] - BSA and Azide free M Flow Cyt, FuncS, IHC-Fr Ms Ms 25244 M IHC-P, WB MHC Class 1 H2 Db antibody [28-14-8] (FITC) M Flow Cyt Ms Ms 25056 Host Species cross reactivity MHC Class 1 H2 Db antibody [KH95] M Flow Cyt, FuncS, WB Ms Ms 64373 Ms Hu, RecFrag MHC Class I H2 Dd antibody [34-2-12] M Flow Cyt, FuncS, IHC-Fr, IP Ms Ms 64368 MHC Class I H2 Dk antibody [15-5-5.3] M Flow Cyt Ms Ms 25216 MHC Class II antigens are a valuable tool for studying T helper cell interactions with class II MHC Class I H2 Kb antibody P ELISA, WB Rb Ms 93364 positive antigen presenting cells, as well as the MHC Class I H2 Kd + Dd + q + u + v antibody [1.B.552] (FITC) M Flow Cyt, IP Ms Ms 62228 development of T helper cells since the antigen is present on stromal cells in the thymus. -

Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases
antibodies Review Targeting Dendritic Cells with Antigen-Delivering Antibodies for Amelioration of Autoimmunity in Animal Models of Multiple Sclerosis and Other Autoimmune Diseases Courtney A. Iberg and Daniel Hawiger * Department of Molecular Microbiology and Immunology, Saint Louis University School of Medicine, Doisy Research Center, 1205 Carr Lane, St. Louis, MO 63104, USA; [email protected] * Correspondence: [email protected] Received: 31 March 2020; Accepted: 30 April 2020; Published: 15 June 2020 Abstract: The specific targeting of dendritic cells (DCs) using antigen-delivering antibodies has been established to be a highly efficient protocol for the induction of tolerance and protection from autoimmune processes in experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS), as well as in some other animal disease models. As the specific mechanisms of such induced tolerance are being investigated, the newly gained insights may also possibly help to design effective treatments for patients. Here we review approaches applied for the amelioration of autoimmunity in animal models based on antibody-mediated targeting of self-antigens to DCs. Further, we discuss relevant mechanisms of immunological tolerance that underlie such approaches, and we also offer some future perspectives for the application of similar methods in certain related disease settings such as transplantation. Keywords: dendritic cells; tolerance; antigen targeting; chimeric antibodies; autoimmunity; multiple sclerosis; diabetes 1. Introduction Over one hundred years ago, Paul Ehrlich coined the term “horror autotoxicus” to define an immune attack against an organism’s healthy tissues [1]. Since then, our knowledge of the complex mechanisms of the immune system as well as our understanding of the pathogenesis of specific autoimmune diseases have grown tremendously. -

Eosinophil Response Against Classical and Emerging
REVIEWS Eosinophil Response Against Classical and Emerging Respiratory Viruses: COVID-19 Rodrigo-Muñoz JM1,2, Sastre B1,2, Cañas JA1,2, Gil-Martínez M1, Redondo N1, del Pozo V1,2 1Immunology Department, Instituto de Investigación Sanitaria (IIS) Fundación Jiménez Díaz, Madrid, Spain 2CIBER de Enfermedades Respiratorias (CIBERES), Madrid, Spain J Investig Allergol Clin Immunol 2021; Vol. 31(2): 94-107 doi: 10.18176/jiaci.0624 Abstract Eosinophils were discovered more than 140 years ago. These polymorphonuclear leukocytes have a very active metabolism and contain numerous intracellular secretory granules that enable multiple effects on both health and disease status. Classically, eosinophils have been considered important immune cells in the pathogenesis of inflammatory processes (eg, parasitic helminth infections) and allergic or pulmonary diseases (eg, asthma) and are always associated with a type 2 immune response. Furthermore, in recent years, eosinophils have been linked to the immune response by conferring host protection against fungi, bacteria, and viruses, which they recognize through several molecules, such as toll-like receptors and the retinoic acid–inducible gene 1–like receptor. The immune protection provided by eosinophils is exerted through multiple mechanisms and properties. Eosinophils contain numerous cytoplasmatic granules that release cationic proteins, cytokines, chemokines, and other molecules, all of which contribute to their functioning. In addition to the competence of eosinophils as effector cells, their capabilities as antigen-presenting cells enable them to act in multiple situations, thus promoting diverse aspects of the immune response. This review summarizes various aspects of eosinophil biology, with emphasis on the mechanisms used and roles played by eosinophils in host defence against viral infections and response to vaccines. -

Efficient Elimination of Primary B-ALL Cells in Vitro and in Vivo Using A
Open access Original research J Immunother Cancer: first published as 10.1136/jitc-2020-000896 on 11 August 2020. Downloaded from Efficient elimination of primary B- ALL cells in vitro and in vivo using a novel 4- 1BB- based CAR targeting a membrane- distal CD22 epitope 1 1 1 Talia Velasco- Hernandez , Samanta Romina Zanetti , Heleia Roca- Ho, Francisco Gutierrez- Aguera,1 Paolo Petazzi,1 Diego Sánchez- Martínez,1 Oscar Molina,1 Matteo Libero Baroni,1 Jose Luis Fuster,2 Paola Ballerini,3 1,4 5 6,7 Clara Bueno, Narcis Fernandez- Fuentes, Pablo Engel , Pablo Menendez1,4,7,8 To cite: Velasco- Hernandez T, ABSTRACT BACKGROUND Zanetti SR, Roca- Ho H, et al. Background There are few therapeutic options available for B-cell acute lymphoblastic leukemia (B-ALL) Efficient elimination of primary patients with B- cell acute lymphoblastic leukemia (B- ALL) is an aggressive cancer, diagnosed at any age B- ALL cells in vitro and in vivo – using a novel 4- 1BB- based relapsing as CD19 either after chemotherapy or CD19- throughout the lifespan of an individual, CAR targeting a membrane- targeted immunotherapies. CD22- chimeric antigen receptor being the most common malignancy in distal CD22 epitope. Journal (CAR) T cells represent an attractive addition to CD19- CAR children.1 Despite current 5-year disease- + – for ImmunoTherapy of Cancer T cell therapy because they will target both CD22 CD19 free survival rates of ~80%, refractory and 2020;8:e000896. doi:10.1136/ B- ALL relapses and CD19– preleukemic cells. However, jitc-2020-000896 relapsed (R/R) patients have a dismal prog- the immune escape mechanisms from CD22- CAR T cells, nosis.2–7 Adult B- ALL is less frequent, but is and the potential contribution of the epitope binding of the commonly associated with an unfavorable ► Additional material is anti-CD22 single-chain variable fragment (scFv) remain published online only.