Femur/Femoral Neck Load Estimation During Stair Navigation Chen Deng Iowa State University
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Blount, Anteversion and Torsion: What's It All About?
Blount, Anteversion and Torsion: What’s it all about? Arthur B. Meyers, MD Assistant Professor of Radiology Children’s Hospital of WisConsin/ MediCal College of WisConsin Disclosures • Author for Amirsys/Elsevier, reCeiving royalGes Lower Extremity Alignment in Children • Lower extremity rotaGon – Femoral version / Gbial torsion – Normal values & CliniCal indiCaGons – Imaging • Blount disease – Physiologic bowing – Blount disease Lower Extremity RotaGonal Alignment Primarily determined by: 1. Femoral version 2. Tibial torsion 3. PosiGon of the foot Rosenfeld SB. Approach to the Child with in-toeing. Up-to-date. 2/2014 Lower Extremity RotaGonal Alignment Primarily determined by: 1. Femoral version 2. Tibial torsion 3. PosiGon of the foot Rosenfeld SB. Approach to the Child with in-toeing. Up-to-date. 2/2014 Femoral Version The rotaGon of the femoral neCk in relaGon to the long axis of the femur (posterior Condylar axis of the distal femur) Femoral Version The rotaGon of the femoral neCk in relaGon to the long axis of the femur (posterior Condylar axis of the distal femur) Femoral Version The rotaGon of the femoral neCk in relaGon to the long axis of the femur (posterior Condylar axis of the distal femur) Femoral Version The rotaGon of the femoral neCk in relaGon to the long axis of the femur (posterior Condylar axis of the distal femur) Femoral Version The rotaGon of the femoral neCk in relaGon to the long axis of the femur (posterior Condylar axis of the distal femur) Femoral Version The rotaGon of the femoral neCk in relaGon to the long -

Patellofemoral Syndrome: Evaluation & Management Scott Sevinsky MSPT
Patellofemoral Syndrome: Evaluation & Management Scott Sevinsky MSPT What is Patellofemoral Syndrome? Patellofemoral syndrome (PFS) is a term commonly used to describe a condition where the patella ‘tracks’ or glides improperly between the femoral condyles. This improper tracking causes pain in the anterior knee and may lead to degenerative changes or dislocation of the knee cap. To be more precise the term ‘anterior knee pain’ is suggested to encompass all pain-related problems of the anterior part of the knee. By excluding anterior knee pain due to intra-articular pathology, peripatellar tendinitis or bursitis, plica syndromes, Sinding Larsen’s disease, Osgood Schlatter’s disease, neuromas and other rarely occurring pathologies it is suggested that remaining patients with a clinical presentation of anterior knee pain could be diagnosed with PFPS. The term ‘patellofemoral’ is used as no distinction can be made as to which specific structure of the patella or femur is affected. The term ‘chondromalacia patellae’, defined at the beginning of the 20th century to describe pathological changes of the retropatellar cartilage,7,8 was for half a century, used as a synonym for the syndrome of patellofemoral pain. However, several studies during the last 2 decades have shown a poor correlation between articular cartilage damage and the still not well-defined pain mechanism of retropatellar pain. Review of Knee Anatomy 1. Femur – thigh bone; longest bone in the body. · Lateral femoral condyle larger than medial condyle & projects farther anteriorly. · Medial femoral condyle longer anterior to posterior · Distal surfaces are convex · Intercondylar (trochlear) notch: groove in which the patella glides or ‘tracks’ 2. -

Femur Pelvis HIP JOINT Femoral Head in Acetabulum Acetabular
Anatomy of the Hip Joint Overview The hip joint is one of the largest weight-bearing HIP JOINT joints in the body. This ball-and-socket joint allows the leg to move and rotate while keeping the body Femoral head in stable and balanced. Let's take a closer look at the acetabulum main parts of the hip joint's anatomy. Pelvis Bones Two bones meet at the hip joint, the femur and the pelvis. The femur, commonly called the "thighbone," is the longest and heaviest bone of the body. At the top of the femur, positioned on the femoral neck, is the femoral head. This is the "ball" of the hip joint. The other part of the joint – the Femur "socket" – is found in the pelvis. The pelvis is a bone made of three sections: the ilium, the ischium and the pubis. The socket is located where these three sections fuse. The proper name of the socket is the "acetabulum." The head of the femur fits tightly into this cup-shaped cavity. Articular Cartilage The femoral head and the acetabulum are covered Acetabular with a layer of articular cartilage. This tough, smooth tissue protects the bones. It allows them to labrum glide smoothly against each other as the ball moves in the socket. Soft Tissues Several soft tissue structures work together to hold the femoral head securely in place. The acetabulum is surrounded by a ring of cartilage called the "acetabular labrum." This deepens the socket and helps keep the ball from slipping out of alignment. It also acts as a shock absorber. -

Study Guide Medical Terminology by Thea Liza Batan About the Author
Study Guide Medical Terminology By Thea Liza Batan About the Author Thea Liza Batan earned a Master of Science in Nursing Administration in 2007 from Xavier University in Cincinnati, Ohio. She has worked as a staff nurse, nurse instructor, and level department head. She currently works as a simulation coordinator and a free- lance writer specializing in nursing and healthcare. All terms mentioned in this text that are known to be trademarks or service marks have been appropriately capitalized. Use of a term in this text shouldn’t be regarded as affecting the validity of any trademark or service mark. Copyright © 2017 by Penn Foster, Inc. All rights reserved. No part of the material protected by this copyright may be reproduced or utilized in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without permission in writing from the copyright owner. Requests for permission to make copies of any part of the work should be mailed to Copyright Permissions, Penn Foster, 925 Oak Street, Scranton, Pennsylvania 18515. Printed in the United States of America CONTENTS INSTRUCTIONS 1 READING ASSIGNMENTS 3 LESSON 1: THE FUNDAMENTALS OF MEDICAL TERMINOLOGY 5 LESSON 2: DIAGNOSIS, INTERVENTION, AND HUMAN BODY TERMS 28 LESSON 3: MUSCULOSKELETAL, CIRCULATORY, AND RESPIRATORY SYSTEM TERMS 44 LESSON 4: DIGESTIVE, URINARY, AND REPRODUCTIVE SYSTEM TERMS 69 LESSON 5: INTEGUMENTARY, NERVOUS, AND ENDOCRINE S YSTEM TERMS 96 SELF-CHECK ANSWERS 134 © PENN FOSTER, INC. 2017 MEDICAL TERMINOLOGY PAGE III Contents INSTRUCTIONS INTRODUCTION Welcome to your course on medical terminology. You’re taking this course because you’re most likely interested in pursuing a health and science career, which entails proficiencyincommunicatingwithhealthcareprofessionalssuchasphysicians,nurses, or dentists. -

Intracapsular Femoral Neck Fractures—A Surgical Management Algorithm
medicina Review Intracapsular Femoral Neck Fractures—A Surgical Management Algorithm James W. A. Fletcher 1,2,* , Christoph Sommer 3, Henrik Eckardt 4, Matthias Knobe 5 , Boyko Gueorguiev 1 and Karl Stoffel 4 1 AO Research Institute Davos, 7270 Davos, Switzerland; [email protected] 2 Department for Health, University of Bath, Bath BA2 7AY, UK 3 Cantonal Hospital Graubünden, 7000 Chur, Switzerland; [email protected] 4 University Hospital Basel, 4052 Basel, Switzerland; [email protected] (H.E.); [email protected] (K.S.) 5 Department of Orthopaedics and Trauma Surgery, Lucerne Cantonal Hospital, 6000 Lucerne, Switzerland; [email protected] * Correspondence: [email protected] Abstract: Background and Objectives: Femoral neck fractures are common and constitute one of the largest healthcare burdens of the modern age. Fractures within the joint capsule (intracapsular) provide a specific surgical challenge due to the difficulty in predicting rates of bony union and whether the blood supply to the femoral head has been disrupted in a way that would lead to avascular necrosis. Most femoral neck fractures are treated surgically, aiming to maintain mobility, whilst reducing pain and complications associated with prolonged bedrest. Materials and Methods: We performed a narrative review of intracapsular hip fracture management, highlighting the latest advancements in fixation techniques, generating an evidence-based algorithm for their management. Results: Multiple different fracture configurations are encountered within the category of intracapsular Citation: Fletcher, J.W.A.; Sommer, hip fractures, with each pattern having different optimal surgical strategies. Additionally, these C.; Eckardt, H.; Knobe, M.; Gueorguiev, B.; Stoffel, K. injuries typically occur in patients where further procedures due to operative complications are Intracapsular Femoral Neck associated with a considerable increase in mortality, highlighting the need for choosing the correct Fractures—A Surgical Management index operation. -

Evaluation of Union of Neglected Femoral Neck Fractures Treated with Free Fibular Graft
ORIGINAL ARTICLE Evaluation of Union of Neglected Femoral Neck Fractures Treated with Free Fibular Graft Nasir Ali, Muhammad Shahid Riaz, Muhammad Ishaque Khan, Muhammad Rafiq Sabir ABSTRACT Objective To evaluate the frequency of union of neglected femoral neck fractures treated with free fibular graft. Study design Descriptive case series. Place & Department of Orthopedics Bahawal Victoria Hospital Bahawalpur, from April 2009 to Duration of January 2010. study Methodology Patients of neglected femoral neck fracture (one month postinjury) were included in the study. They were operated and internal fixation was done with concellous screws and free fibular graft placed. They were followed till the evidence of radiological union. Results Out of 55 patients there were 40 males and 15 females. Ages ranged from 20 year to 50 year. The duration of injury was from 4 weeks to 6 months. Fifty patients achieved complete union while five patients developed non-union with complaint of pain. There was no wound infection and hardware failure. Conclusion Fracture reduction and internal fixation with use of free fibular graft and concellous screws for neglected femoral neck fractures is the treatment of choice. Key words Bony union, Fracture- femur, Fibular graft. INTRODUCTION: time of injury to seek medical help.7 With delay these Fractures of neck of femur are great challenge to factures usually result in non union. The rate of non orthopaedic surgeons. With increase in life union is between 10-30% for such neglected expectancy and addition of geriatric population to fractures.8,9 Delay in surgery leads to variable degree society, the frequency of fracture neck of femur is of neck absorption, proximal migration of distal increasing day by day.1 Fracture neck of femur in fragment and disuse osteoporosis. -

Luxating Patella
LUXATING PATELLA What is a luxating patella? The patella, or kneecap, is normally located in the center of the knee joint. The term luxating means, “out of place” or “dislocated”. Therefore, a luxating patella is a kneecap that moves out of its normal location. What causes this? The muscles of the thigh attach to the top of the kneecap. There is a ligament, the patellar ligament, running from the bottom of the kneecap to a point on the tibia just below the knee joint. When the thigh muscles contract, force is transmitted through the patella and patellar ligament to a point on the top of the tibia. This results in extension or straightening of the knee. The patella stays in the center of the leg because the point of attachment of the patellar ligament is on the midline and because the patella slides in a groove on the lower end of the femur (the bone between the knee and the hip). The patella luxates because the point of attachment of the patellar ligament is not on the midline of the tibia. It is almost always located too far medial (toward the middle of the body). As the thigh muscles contract, the force is pulled medial. After several months or years of this abnormal movement, the inner side of the groove in the femur wears down. Once the side of the groove wears down, the patella is then free to dislocate. When this occurs, the dog has difficulty bearing weight on the leg. It may learn how to kick the leg and snap the patella back into its normal location. -
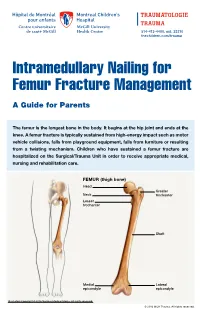
Intramedullary Nailing for Femur Fracture Management a Guide for Parents
514-412-4400, ext. 23310 thechildren.com/trauma Intramedullary Nailing for Femur Fracture Management A Guide for Parents The femur is the longest bone in the body. It begins at the hip joint and ends at the knee. A femur fracture is typically sustained from high-energy impact such as motor vehicle collisions, falls from playground equipment, falls from furniture or resulting from a twisting mechanism. Children who have sustained a femur fracture are hospitalized on the Surgical/Trauma Unit in order to receive appropriate medical, nursing and rehabilitation care. FEMUR (thigh bone) Head Greater Neck trochanter Lesser trochanter Shaft Medial Lateral epicondyle epicondyle Illustration Copyright © 2016 Nucleus Medical Media, All rights reserved. © 2016 MCH Trauma. All rights reserved. FEMUR FRACTURE MANAGEMENT The pediatric Orthopedic Surgeon will assess your child in order to determine the optimal treatment method. Treatment goals include: achieving proper bone realignment, rapid healing, and the return to normal daily activities. The treatment method chosen is primarily based on the child’s age but also taken into consideration are: fracture type, location and other injuries sustained if applicable. Prior to the surgery, your child may be placed in skin traction. This will ensure the bone is in an optimal healing position until it is surgically repaired. Occasionally, traction may be used for a longer period of time. The surgeon will determine if this management is needed based on the specific fracture type and/or location. ELASTIC/FLEXIBLE INTRAMEDULLARY NAILING This surgery is performed by the Orthopedic Surgeon in the Operating Room under general anesthesia. The surgeon will usually make two small incisions near the knee joint in order to insert two flexible titanium rods (intramedullary nails) Flexible through the femur. -

Unilateral Proximal Focal Femoral Deficiency, Fibular Aplasia, Tibial
The Egyptian Journal of Medical Human Genetics (2014) 15, 299–303 Ain Shams University The Egyptian Journal of Medical Human Genetics www.ejmhg.eg.net www.sciencedirect.com CASE REPORT Unilateral proximal focal femoral deficiency, fibular aplasia, tibial campomelia and oligosyndactyly in an Egyptian child – Probable FFU syndrome Rabah M. Shawky a,*, Heba Salah Abd Elkhalek a, Shaimaa Gad a, Shaimaa Abdelsattar Mohammad b a Pediatric Department, Genetics Unit, Ain Shams University, Egypt b Radio Diagnosis Department, Ain Shams University, Egypt Received 2 March 2014; accepted 18 March 2014 Available online 30 April 2014 KEYWORDS Abstract We report a fifteen month old Egyptian male child, the third in order of birth of healthy Short femur; non consanguineous parents, who has normal mentality, normal upper limbs and left lower limb. Limb anomaly; The right lower limb has short femur, and tibia with anterior bowing, and an overlying skin dimple. FFU syndrome; The right foot has also oligosyndactyly (three toes), and the foot is in vulgus position. There is lim- Proximal focal femoral ited abduction at the hip joint, full flexion and extension at the knee, limited dorsiflexion and plan- deficiency; tar flexion at the ankle joint. The X-ray of the lower limb and pelvis shows proximal focal femoral Fibular aplasia; deficiency, absent right fibula with shortening of the right tibia and anterior bowing of its distal Tibial campomelia; third. The acetabulum is shallow. He has a family history of congenital cyanotic heart disease. Oligosyndactyly Our patient represents most probably the first case of femur fibula ulna syndrome (FFU) in Egypt with unilateral right leg affection. -
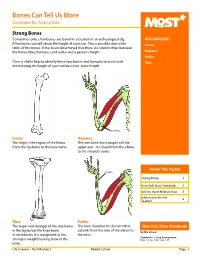
Bones Can Tell Us More Compiled By: Nancy Volk
Bones Can Tell Us More Compiled By: Nancy Volk Strong Bones Sometimes only a few bones are found in a location in an archeological dig. VOCABULARY A few bones can tell about the height of a person. This is possible due to the Femur ratios of the bones. It has been determined that there are relationships between the femur, tibia, humerus, and radius and a person’s height. Humerus Radius Here is a little help to identify these four bones and formulas to assist with Tibia determining the height of a person based on bone length. Humerus Femur: Humerus: The thigh is the region of the femur. The arm bone most people call the From the hip bone to the knee bone. upper arm. It is found from the elbow to the shoulder joints. Inside This Packet Radius Strong Bones 1 New York State Standards 1 Activity: Bone Relationships 2 Information for the Teacher 4 Tibia: Radius: The larger and stronger of the two bones The bone found in the forearm that New York State Standards in the leg below the knee bone. extends from the side of the elbow to Middle School In vertebrates It is recognized as the the wrist. Standard 4: Living Environment strongest weight bearing bone in the Idea 1: 1.2a, 1.2b, 1.2e, 1.2f body. Life Sciences - Post Module 3 Middle School Page 1 Activity: Bone Relationships MATERIALS NEEDED Skeleton Formulas: Tape Measure Bone relationship is represented by the following formulas: Directions and formulas P represents the person’s height. The last letter of each formula stands for the Calculator known length of the bone (femur, tibia, humerus, or radius) through measurement. -

Normative Values for Femoral Length, Tibial Length, Andthe Femorotibial
Article Normative Values for Femoral Length, Tibial Length, and the Femorotibial Ratio in Adults Using Standing Full-Length Radiography Stuart A Aitken MaineGeneral Medical Center, 35 Medical Center Parkway, Augusta, ME 04330, USA; [email protected] Abstract: Knowledge of the normal length and skeletal proportions of the lower limb is required as part of the evaluation of limb length discrepancy. When measuring limb length, modern standing full-length digital radiographs confer a level of clinical accuracy interchangeable with that of CT imaging. This study reports a set of normative values for lower limb length using the standing full-length radiographs of 753 patients (61% male). Lower limb length, femoral length, tibial length, and the femorotibial ratio were measured in 1077 limbs. The reliability of the measurement method was tested using the intra-class correlation (ICC) of agreement between three observers. The mean length of 1077 lower limbs was 89.0 cm (range 70.2 to 103.9 cm). Mean femoral length was 50.0 cm (39.3 to 58.4 cm) and tibial length was 39.0 cm (30.8 to 46.5 cm). The median side-to-side difference was 0.4 cm (0.2 to 0.7, max 1.8 cm) between 324 paired limbs. The mean ratio of femoral length to tibial length for the study population was 1.28:1 (range 1.16 to 1.39). A moderately strong inverse linear relationship (r = −0.35, p < 0.001, Pearson’s) was identified between tibial length and the Citation: Aitken, S.A. Normative corresponding femorotibial ratio. -

Congenital Abnormalities of the Femur
Arch Dis Child: first published as 10.1136/adc.36.188.410 on 1 August 1961. Downloaded from CONGENITAL ABNORMALITIES OF THE FEMUR BY P. A. RING From the Royal College of Surgeons (RECEIVED FOR PUBLICATION NOVEMBER 25, 1960) Congenital defects of the femur vary from simple tion of its incidence is difficult to obtain, but it hypoplasia of the bone to complete absence. appears to be the commonest congenital defect Classification of these defects has been suggested causing major abnormalities of limb growth. by Nilsonne (1928) and by Mouchet and Ibos (1928), but neither has met with general acceptance. In more recent years Golding (1939, 1948) has demon- Clinical Features strated the close association of the short femur with There is no evidence that this is a familial disorder, congenital coxa vara, and has emphasized that and careful inquiry of the parents has revealed no these are variations of the same underlying abnor- evidence of other congenital disorders within the mality. The clinical distinction between the various immediate family. The history of the pregnancy types of femoral defect is important as a guide to and delivery has failed to indicate any significant the prognosis of limb development. infection or abnormality at this time. In most From an examination of patients with congenital patients the abnormality is apparent at birth, but abnormalities of the femur the following classifica- where the inequality of leg length is slight, the by copyright. tion is suggested: diagnosis may not be made until the child begins to 1. Simple femoral hypoplasia. walk. To ordinary clinical testing the abnormality 2.