2016 Fisheries Investigations in Lakes and Streams
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Chaelundi National Park and Chaelundi State Conservation Area
CHAELUNDI NATIONAL PARK AND CHAELUNDI STATE CONSERVATION AREA PLAN OF MANAGEMENT NSW National Parks and Wildlife Service Part of the Department of Environment and Climate Change NSW May 2009 This plan of management was adopted by the Minister for Climate Change and the Environment on 29th May 2009. Acknowledgments The plan of management is based on a draft plan prepared by staff of the North Coast Region of the NSW National Parks and Wildlife Service (NPWS) with the assistance of staff from other sections and divisions in the Department of Environment and Climate Change (DECC). Valuable information and comments provided by DECC specialists, the Regional Advisory Committee, and members of the public who participated in consultation workshops or contributed to the planning process in any way are gratefully acknowledged. The NPWS acknowledges that these parks are within the traditional country of the Gumbaynggirr Aboriginal people. Cover photographs by Aaron Harber, NPWS. Inquiries about these parks or this plan of management should be directed to the Ranger at the NPWS Dorrigo Plateau Area Office, Rainforest Centre, Dorrigo National Park, Dome Road, Dorrigo NSW 2453 or by telephone on (02) 6657 2309. © Department of Environment and Climate Change NSW 2009. ISBN 978 1 74232 382 4 DECC 2009/512 FOREWORD Chaelundi National Park and Chaelundi State Conservation Area are located approximately 45 kilometres south west of Grafton and 10 kilometres west of Nymboida in northern NSW. Together the parks cover an area of approximately 20,796 hectares. Chaelundi National Park and State Conservation Area protect the old growth forest communities and other important habitat, and plants and animals of high conservation value, including endangered species and the regionally significant brush-tailed rock wallaby. -

By Michael G. Zalants Water-Resources Investigations
LOW-FLOW CHARACTERISTICS OF NATURAL STREAMS IN THE BLUE RIDGE, PIEDMONT, AND UPPER COASTAL PLAIN PHYSIOGRAPHIC PROVINCES OF SOUTH CAROLINA By Michael G. Zalants U.S. GEOLOGICAL SURVEY Water-Resources Investigations Report 90-4188 Prepared in cooperation with the SOUTH CAROLINA DEPARTMENT OF HEALTH AND ENVIRONMENTAL CONTROL Columbia, South Carolina 1991 U.S. DEPARTMENT OF THE INTERIOR MANUEL LUGAN, JR., Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director For additional information Copies of this report may be write to: purchased from: District Chief U.S. Geological Survey U.S. Geological Survey Books and Open-File Reports 720 Gracern Road Feoteral Center, Bldg. 810 Stephenson Center, Suite 129 Box| 25425 Columbia, South Carolina 29210 Denver, Colorado 80225 CONTENTS Page Abstract ............................................................. 1 Introduction ......................................................... 2 Purpose and scope ............................................... 2 Previous studies ................................................ 2 Description of the study area ................................... 3 Streamflow data compilation .......................................... 3 Continuous-record stations ...................................... 3 Partial-record stations ......................................... 6 Low-flow characteristics ............................................. 7 Continuous-record stations ...................................... 7 Partial-record stations ......................................... 8 Ungaged -

Piedmont Ecoregion Aquatic Habitats
Piedmont Ecoregion Aquatic Habitats Description and Location The piedmont ecoregion extends south of Blue Ridge to the fall line near Columbia, South Carolina and from the Savannah River east to the Pee Dee River. Encompassing 24 counties and 10,788 square miles, the piedmont is the largest physiographic province in South Carolina. The piedmont is an area with gently rolling hills dissected by narrow stream and river valleys. Forests, farms and orchards Pee Dee-Piedmont EDU dominate most of the land. Elevations Santee-Piedmont EDU Savannah-Piedmont EDU range from 375 to 1,000 feet. The Piedmont Ecoregion cuts across the top of three major South Carolina drainages, the Savannah, the Santee and the Pee Dee, forming three ecobasins: the Savannah-Piedmont, Santee-Piedmont and Pee Dee-Piedmont. Savannah-Piedmont Ecobasin The Savannah River drainage originates in the mountains of North Carolina and Georgia. The Savannah River flows southeast along the border of South Carolina and Georgia through the piedmont for approximately 131 miles on its way to the Atlantic Ocean. Major tributaries to the Savannah River in the South Carolina portion of this ecobasin include the Tugaloo River, Seneca River, Chauga River, Rocky River, Little River and Stevens Creek. The ecobasin encompasses 36 watersheds and approximately 2,879 square miles. The vast majority of the land is privately owned with only 239 square miles protected by federal, state and private entities. Most of the protected land (192 square miles) occurs in Sumter National Forest. The ecobasin contains 3,328 miles of lotic habitat with 143 square miles of impoundments. -

Koala Conservation Status in New South Wales Biolink Koala Conservation Review
koala conservation status in new south wales Biolink koala conservation review Table of Contents 1. EXECUTIVE SUMMARY ............................................................................................... 3 2. INTRODUCTION ............................................................................................................ 6 3. DESCRIPTION OF THE NSW POPULATION .............................................................. 6 Current distribution ............................................................................................................... 6 Size of NSW koala population .............................................................................................. 8 4. INFORMING CHANGES TO POPULATION ESTIMATES ....................................... 12 Bionet Records and Published Reports ............................................................................... 15 Methods – Bionet records ............................................................................................... 15 Methods – available reports ............................................................................................ 15 Results ............................................................................................................................ 16 The 2019 Fires .................................................................................................................... 22 Methods ......................................................................................................................... -

Bindarri National Park and State Conservation Area Fire Management Strategy
North Coast Region Index Locality Risk Management Information Bushfire Suppression Sheas Nob SF Byrnes Scrub NR 488000m.E 89 490 91 92 93 94 95 96 97 498000m.E Bindarri National Park and State Chaelundi NP Sherwood NR Coffs Coast RP vv Mound Of Stones Bel MOLETON MOONEE BEACH la Sp Mo Conglomerate SF Wedding Bells SF ur Rd r Cemetery to Conservation Area 25k mapsheet 25k mapsheet v n Helipad Information s Nymboi-Binderay NP R 94371S 95374S Woolgoolga d Fire Management Strategy (Type 2) Kangaroo River SF (! Name Easting Northing Lat_DMS Long_DMSv N v N vvv ! ! ! Clouds Creek SF Potential Helipad 1 491005 6648654 30d 17m 37v S 152d 54m 23s E 2005 000m. Potential Helipad 2 490404 6652197 30d 15m 42 S 152d 54m 01s E ! ! ! ! 000m. 57 v ! ! 57 66 Bagawa SF Northern HMZ 66 Potential Helipad 3 495401 6652185 30d 15m 43 S 152d 57m 08s E Sheet 1 of 1 v ! ! ! ! ! ! ! ! ! ! ! ! ! Fire Hut FL1 L Lower Bucca SF Dry Creek HMZ v o FL2 This strategy should be used in conjunction with aerial photography and field reconnaissance w FL2 a Wild Cattle Creek SF n ! ! ! ! ! ! ! ! Langleys Rd HMZ n Moonee Beach NR a during incidents and the development of incident action plans. Coramba NR v R FL2 FL2 d ! ! ! ! ! ! ! ! Moonpar SF Nana Creek SF L These data are not guaranteed to be free from error or omission. The NSW National Parks and Wildlife and its employees a Mirum Creek HMZ ! ! ! ! ! ! ! ! n FL2 v g Nymboi-Binderay SCA le disclaim liability for any act done on the information in the data and any consequences of such acts or omissions. -

Introduction the Need for New Reserves
Koala Strategy Submissions, PO Box A290, Sydney South NSW 1232, [email protected] CC. [email protected] 2nd March 2017. NPA SUBMISSION: WHOLE OF GOVERNMENT KOALA STRATEGY; SAVING OUR SPECIES DRAFT STRATEGY AND REVIEW OF STATE ENVIRONMENT PLANNING POLICY 44—KOALA HABITAT PROTECTION Introduction The National Parks Association of NSW (NPA), established in 1957, is a community-based organisation with over 20,000 supporters from rural, remote and urban areas across the state. NPA promotes nature conservation and evidence- based natural resource management. We have a particular interest in the protection of the State’s biodiversity and supporting ecological processes, both within and outside of the formal conservation reserve system. NPA has a long history of engagement with both government and non-government organisations on issues of park management. NPA appreciates the opportunity to comment on the whole of government koala strategy (the strategy), the Saving Our Species iconic koala project (the SOS project) and the Explanation of intended effect: State Environment and Planning Policy 44 (Koala Habitat Protection) (SEPP 44). Please note, NPA made a submission on SEPP 44 in late 2016 prior to the deadline being extended which we have reattached at the end of this document (Appendix 1). NPA was also consulted on the SOS programme in August 2016, which we greatly appreciated. We provided OEH with feedback on the programme at that time, which also contained an exploration of issues facing koalas, which we have reattached in Appendix 2. NPA has had significant input to the development of the Stand Up For Nature (SUFN) submission to this consultation. -

Notes for Cyclists Mountain Bikes on the BNT
Notes for Cyclists Mountain Bikes on the BNT Please note: This information is for those intending to take mountain bikes out on the Bicentennial National Trail and offers suggestions for cyclists to avoid those sections of the Trail unsuitable for bikes. The information provided here is of a general nature and should be read in conjunction with the Guidebooks, Guidebook updates and road maps. Cyclists should always check back on the website for the most recent information on Trail conditions. A friendly reminder that all Trail users are self reliant trekkers responsible for all your needs and the decisions you make. Just like the Trail, this information sheet is an ongoing work in progress - users of these notes, and the Trail, are invited to offer their own opinions and suggestions for updating this information. The best parts of the National Trail for walkers and trekkers with animals are the rugged sections through wilderness. The Trail has been designed to provide a balance between these rugged remote areas and easier more accessible parts along quiet roads and tracks through the countryside. For cyclists, unless you are especially masochistic, you will find it necessary to detour around the particularly difficult sections. Bypassing sections of the BNT unsuitable for bikes usually involves public roads, mostly quiet back roads, so landowner permission for access is not a problem. As the detours are generally close to the BNT, the spirit of the BNT concept is not lost. However, this has left some cyclists with the impression that the Bicentennial National Trail is much less challenging than it really is. -
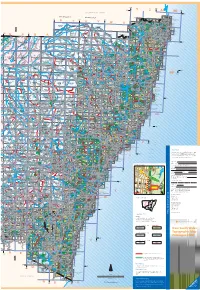
New South Wales Topographic Map Catalogue 2008
° 152 153° Y 154° 28° Z AA BB QUEENSLAND 28° Coolangatta SPRING- CURRU- TWEED HEAD WARWICK BROOK MBIN SFingal Head AMG / MGA ZONE 55 AMG /MGA ZONE 56 MT LINDESAY MURWILLUMBAH 9641-4S 9341 Terranora TWEED HEADS KILLAR- WILSON M 9441 LAM- TYAL- S MOUNT OUNT PALEN 9541Tumbulgum Kingscliff 151° NEY PEAK LINDE- COUGAL INGTON GUM MURWILLUMBAH 9641 ° ° CLUNIE CREEK CUDGEN 149 150 KOREELAH N P SAY TYALGUMChillingham9541-2N Condong Bogangar Woodenbong 96 Legume BORDER RANGES 9541-3-N I 41-3N N P Old Hastings Point S EungellaTWEEDI ELBOW VALLEY KOREE TOOLOOM Grevillia MOOBALL NP V LAH I T WOOD MT. WARNING U N P ENBONG Uki Pottsville Beach G W 9341-3-S REVILLIA BRAYS N P 9341-2-S CREEK 9441 BURRINGB I POTTSVIL X -3-S AI R LE 9441-2-S Mount I Grevillia 9541-3S 9541-2 Mooball Goondiwindi Urbenville Lion S 9641-3S TOONUMBAR N P MEBBIN N P MOUNT I MARYLAND Kunghur JERUSALEM N P Wiangaree I N P WYLIE CREEK TOOLOOM NIGHTCAP Ocean Shores GRADULE BOOMI CAPEEN AFTERLE Mullumbimby BOGGABILLA YELARBO 9340-4-N 9340 E NIMBIN N P I 8740-N I N -1-N 9440-4-N HUONBROOK BRUNSWICK 8840-N 9440-1-N I 8940-N 9040-N 9540-4N Nimbin 9540-1NBYRON I Toonumbar I HEA Liston Tooloom GOONENGERRY NP DS I Rivertree Old Bonalbo RICHMOND KYOGLE Cawongla I 9640-4N Kyogle I Boomi I LISTON RANGE N P PADDYS FLATYABBRA BONALBO LISMORERosebank Federal ETTRIC I Byron Bay GO I N P K ONDIWINDI YETMAN 9340-4-S 9340-1-S LARNOOK The D BYRON BAY S R A TEXAS 9440-4-S UNOON BI YRI ON BA STANTHORP Paddys Flat 9440-1-S Cedar 9540 Channon I Y 8940 I E DRAKE -4S 9540-1S Suffolk Park 9040 BON Dryaaba -
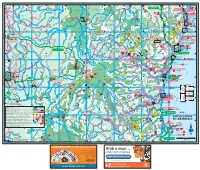
Coffs Coast Regional
TO GLEN TO NYMBOIDA TO McPHERSONS TO GRAFTON INNES 28km ABCDE3km CROSSING 26km FTO GRAFTON 36km 41km G YURAYGIR HORTONS NAT PK CK NAT N WARRA 152º50'E Red Rock 152º10'E 153º10'E 151º50'E 152º00'E 153º00'E 152º20'E 152º30'E 152º40'E NAT PK (locality) RES o TOURIST DRIVE 19 rt Kookabookra h YARRAWARRA CHAELUNDI KA 30º00'S SHEAS NG ABORIGINAL 1 RED ROCK STATE FOREST 996 CHAELUNDI AR GUY FAWKES NOB SF 803 O CULTURAL CENTRE NATIONAL O HOLIDAY PARK Ben Lomond PARK RIVER k k e Towallum e e r e P Y r R Corindi Beach A C D IV C E Glenreagh SHERWOOD CORINDI BEACH R R W Fawkes BYRNES NAT RES H HOLIDAY PARK CLOUDS CREEK SCRUB Upper G LLANGOTHLIN I 10 O Corindi RD NAT RES H For detail see Map 8 N R LAKE The T Chaelundi Ck a E (locality) A MARENGO A Arrawarra Platypus are found in most RD l R W R Rest Area Junction l SF 318 a A Backwater K pools along the river 4WD beach access 12 LITTLE R w 1 O E KANGAROO Coast 1 u WEDDING LLANGOTHLIN O V B um I d BELLS Mullaway A s j NAT RES s R a K po RIVER CONGLOMERATE O h Woolgoolga Beach O Clouds Creek 12 O CHAELUNDI RD 4WD beach access K River an ST CONS Railway b NYMBOI - BINDERAY Railway Oban O SF 21 SF 349 For detail Woolgoolga AREA NATIONAL PARK GENTLE ANNIE see Map 3 s SF 111 d Little FOREST WAY n a Ma ELLIS SF 831 N uc l Riv re Misty Creek O B ca SOLITARY w er n T RD o g Lookout Vista Point F Cod Hole N o Woolgoolga ISLANDS Llangothlin MIST A Bucca Wards Mistake R A N Nana Glen Creek Picnic AKE Marengo Falls G y Hearnes Lake (locality) D m BAGAWA SF 30 13 Area NATIONAL I R Lower b WILD I S O V o Bucca Sandy Beach GARA D Ck i MT E For detail B d R CATTLE BUSHMANS R Sandy Beach A PARK HYLAND M a RD see Map 9 W CREEK Moleton SF NAT RES Y 11 Fiddamans Beach 318 N SF 488 LOWER M Billys Creek Platypus SF Emerald Beach 30º10'S A BUCCA le T 11 oy R Flat 536 Look at Me Now f 10 r DR e E S Headland b SF 29 E A N R A R 5 k G N Ck O MOONEE BEACH e O FO G R E F e 23 EST MOTHER OF DUCKS r Dundarrabin NATURE RESERVE P Norman Jolly R. -

Chaelundi National Park and State Conservation Area Fire Management Strategy
Locality Communications Information Contact Information Operational Guidelines General Guidelines continued Fire History (time since fire) North Coast Region Service Channel Location and Comments Agency Position / Location Phone Mobile/Fax/Pager Refer to NPWS “Strategy for Fire Management 2003” and NPWS “Fire Management Manual 2004”. Command & Control • The first combatant agency on site may assume control of the fire, but then must Brief all personnel involved in suppression operations on the following issues: ensure the relevant land management agency is notified promptly. NPWS - VHF (Dorrigo) 23 & 25 Reverse channels 67& 69 (Dorrigo) National Parks & Regional Duty Officer – North Coast 0428 345 789 016 301161 (pager) (NPWS FMM 4.2) Chaelundi National Park and Resource Guidelines • On the arrival of other combatant agencies, the initial incident controller will Some dead spots; can delink Ch. 23 if required Wildlife: Dorrigo Plateau Area Manager 02 6657 2309 0427 109030 Aboriginal Cultural • Aboriginal site locations are not shown on the exhibited strategy for confidentiality. consult with regard to the ongoing command, control and incident management State Conservation Area NPWS – VHF (Grafton) 9 & 10 Reverse channels 53 & 54 (Grafton) North Coast Fire Management Officer - North Coast 02 6641 1500 0427 250122 Heritage Management Liaise with the relevant DEC Cultural Heritage Conservation Officer to check for team requirements as per the relevant BFMC Plan of Operations. NPWS - VHF (Portable Repeater) 14 Orange Contact Dorrigo Plateau Area office to deploy. Regional Operations Coordinator - Nth Coast 02 6641 1500 0427 165785 (NPWS FMM 4.11) sites & appropriate fire prescriptions. Containment Lines • Construction of new containment lines should be avoided (i.e. -
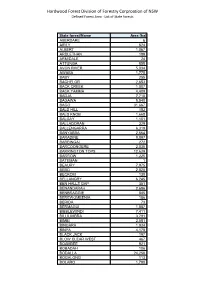
Defined Forest Area - List of State Forests
Hardwood Forest Division of Forestry Corproation of NSW Defined Forest Area - List of State forests State forest/Name Area (ha) ABERDARE 6 AIRLY 624 ALBERT 1,067 ARDLETHAN 189 ARMIDALE 24 ATTUNGA 858 AVON RIVER 5,034 AWABA 1,775 BABY 255 BACHELOR 2,653 BACK CREEK 1,007 BACK YAMMA 4,409 BADJA 7,715 BAGAWA 5,540 BAGO 31,667 BALD HILL 152 BALD KNOB 1,668 BALGAY 1,101 BALLADORAN 329 BALLENGARRA 6,319 BANYABBA 2,664 BARADINE 9,887 BARBINGAL 272 BARCOONGERE 2,035 BARRINGTON TOPS 12,629 BARROW 1,225 BATEMAN 1 BEAURY 7,975 BEBO 2,820 BECKOM 138 BELLANGRY 6,245 BEN HALLS GAP 351 BENANDARAH 2,686 BENBRAGGIE 845 BEREWOMBENIA 186 BERIDA 73 BERMAGUI 1,887 BIBBLEWINDI 7,411 BILLILIMBRA 3,791 BIMBI 2,581 BINGARA 1,934 BINYA 4,178 BLACK JACK 197 BLOW CLEAR WEST 467 BOAMBEE 921 BOBADAH 106 BODALLA 24,258 BOGALONG 113 BOLARO 1,790 Hardwood Forest Division of Forestry Corproation of NSW Defined Forest Area - List of State forests State forest/Name Area (ha) BOM BOM 886 BOMBALA 349 BONALBO 2,496 BONDI 6,552 BONDO 15,199 BOOBEROI 833 BOOKOOKOORARA 916 BOONA 1,184 BOONANGHI 3,807 BOONOO 4,253 BOORABEE 1,120 BOOROOK 3,035 BOUNDARY CREEK 2,532 BOURBAH 623 BOWMAN 3,205 BOXALLS 402 BOYBEN 2,569 BOYNE 6,259 BRAEMAR 2,024 BRASSEY 744 BREEZA 1,361 BRETTS 735 BRIL BRIL 2,364 BROKEN BAGO 4,095 BROKEN RANGE 409 BROOKONG 333 BROTHER 6,539 BRUCES CREEK 714 BUCKENBOWRA 5,202 BUCKINGBONG 11,670 BUCKRA BENDINNI 1,763 BULAHDELAH 8,751 BULBODNEY 2,387 BULGA 14,568 BULLS GROUND 2,144 BUNGABBEE 1,097 BUNGANBIL 465 BUNGAWALBIN 1,212 BUNGONGO 1,065 BURRAWAN 2,236 BUTTERLEAF 1,748 -
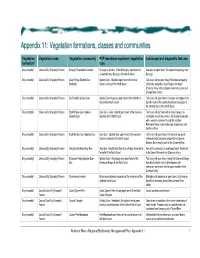
Vegetation Formations, Classes and Communities
Appendix 11: Vegetatiion formatiions, cllasses and communiities Vegetation Vegetation class Vegetation community PVP developer equivalent vegetation Landscape and diagnostic features formation* type Dry sclerophyll Clarence Dry Sclerophyll Forests Baryulgil Serpentinite Complex Eucalyptus ophitica - White Mahogany open forest on Low open or open forest. On serpentinite geology near serpentinite near Baryulgil of the North Coast Baryulgil. Dry sclerophyll Clarence Dry Sclerophyll Forests Coast Range Spotted Gum- Spotted Gum - Blackbutt open forest of the lower Tall to very tall dry open forest. Restricted and patchy Blackbutt Clarence Valley of the North Coast distribution along the Coast Range in the lower Clarence Valley, with a disjunct western occurrence in Grange State Forest. Dry sclerophyll Clarence Dry Sclerophyll Forests Dry Foothills Spotted Gum Spotted Gum dry grassy open forest of the foothills of Tall to very tall open forest. On slopes and ridges of dry the northern North Coast foothills areas of the coastal hinterland and gorges of the northern parts of the North Coast. Dry sclerophyll Clarence Dry Sclerophyll Forests Foothill Grey Gum-Ironbark- Grey Gum - Grey Ironbark open forest of the Clarence Tall to very tall dry forest with a mixed canopy. On Spotted Gum lowlands of the North Coast sandstone and siliceous soils in the Clarence lowlands with a western extension through the southern Richmond Range inland to Ewingar State Forest and the Mann River. Dry sclerophyll Clarence Dry Sclerophyll Forests Foothills Grey Gum-Spotted Gum Grey Gum - Spotted Gum open forest of the southern Tall to very tall open forest. On high and low quartz Clarence lowlands of the North Coast sediments in the southern portion of the Clarence- Moreton Basin mainly south of the Clarence River.