Pain in Acquired Inflammatory Demyelinating Polyneuropathies Siddarth Thakura, Robert H
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
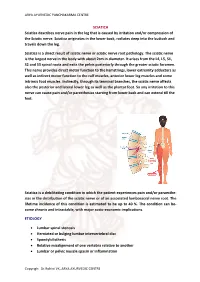
SCIATICA Sciatica Describes Nerve Pain in the Leg That Is Caused by Irritation And/Or Compression of the Sciatic Nerve
ARYA AYURVEDIC PANCHAKARMA CENTRE SCIATICA Sciatica describes nerve pain in the leg that is caused by irritation and/or compression of the Sciatic nerve. Sciatica originates in the lower back, radiates deep into the buttock and travels down the leg. Sciatica is a direct result of sciatic nerve or sciatic nerve root pathology. The sciatic nerve is the largest nerve in the body with about 2cm in diameter. It arises from the L4, L5, S1, S2 and S3 spinal roots and exits the pelvis posteriorly through the greater sciatic foramen. This nerve provides direct motor function to the hamstrings, lower extremity adductors as well as indirect motor function to the calf muscles, anterior lower leg muscles and some intrinsic foot muscles. Indirectly, through its terminal branches, the sciatic nerve affects also the posterior and lateral lower leg as well as the plantar foot. So any irritation to this nerve can cause pain and/or paresthesias starting from lower back and can extend till the feet. Sciatica is a debilitating condition in which the patient experiences pain and/or paraesthe- sias in the distribution of the sciatic nerve or of an associated lumbosacral nerve root. The lifetime incidence of this condition is estimated to be up to 40 %. The condition can be- come chronic and intractable, with major socio-economic implications. ETIOLOGY • Lumbar spinal stenosis • Herniated or bulging lumbar intervertebral disc • Spondylolisthesis • Relative misalignment of one vertebra relative to another • Lumbar or pelvic muscle spasm or inflammation Copyrigh: Dr.Rohini VK, ARYA AYURVEDIC CENTRE ARYA AYURVEDIC PANCHAKARMA CENTRE • Spinal or Paraspinal masses included malignancy, epidural haematoma or epidural abscess • Lumbar degenerative disc disease • Sacroiliac joint dysfunction EPIDEMIOLOGY GENDER: There appears to be no gender predominance. -

Radiculopathy Vs. Spinal Stenosis: Evocative Electrodiagnosis Identifies the Main Pain Generator
Functional Electromyography Loren M. Fishman · Allen N. Wilkins Functional Electromyography Provocative Maneuvers in Electrodiagnosis 123 Loren M. Fishman, MD Allen N. Wilkins, MD College of Physicians & Surgeons Manhattan Physical Medicine Columbia University and Rehabilitation New York, NY 10028, USA New York, NY 10013, USA [email protected] ISBN 978-1-60761-019-9 e-ISBN 978-1-60761-020-5 DOI 10.1007/978-1-60761-020-5 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010935087 © Springer Science+Business Media, LLC 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein. -

Piriformis Syndrome: the Literal “Pain in My Butt” Chelsea Smith, PTA
Piriformis Syndrome: the literal “pain in my butt” Chelsea Smith, PTA Aside from the monotony of day-to-day pains and annoyances, piriformis syndrome is the literal “pain in my butt” that may not go away with sending the kids to grandmas and often takes the form of sciatica. Many individuals with pain in the buttock that radiates down the leg are experiencing a form of sciatica caused by irritation of the spinal nerves in or near the lumbar spine (1). Other times though, the nerve irritation is not in the spine but further down the leg due to a pesky muscle called the piriformis, hence “piriformis syndrome”. The piriformis muscle is a flat, pyramidal-shaped muscle that originates from the front surface of the sacrum and the joint capsule of the sacroiliac joint (SI joint) and is located deep in the gluteal tissue (2). The piriformis travels through the greater sciatic foramen and attaches to the upper surface of the greater trochanter (or top of the hip bone) while the sciatic nerve runs under (and sometimes through) the piriformis muscle as it exits the pelvis. Due to this close proximity between the piriformis muscle and the sciatic nerve, if there is excessive tension (tightness), spasm, or inflammation of the piriformis muscle this can cause irritation to the sciatic nerve leading to symptoms of sciatica (pain down the leg) (1). Activities like sitting on hard surfaces, crouching down, walking or running for long distances, and climbing stairs can all increase symptoms (2) with the most common symptom being tenderness along the piriformis muscle (deep in the gluteal region) upon palpation. -

Piriformis Syndrome Is Overdiagnosed 11 Robert A
American Association of Neuromuscular & Electrodiagnostic Medicine AANEM CROSSFIRE: CONTROVERSIES IN NEUROMUSCULAR AND ELECTRODIAGNOSTIC MEDICINE Loren M. Fishman, MD, B.Phil Robert A.Werner, MD, MS Scott J. Primack, DO Willam S. Pease, MD Ernest W. Johnson, MD Lawrence R. Robinson, MD 2005 AANEM COURSE F AANEM 52ND Annual Scientific Meeting Monterey, California CROSSFIRE: Controversies in Neuromuscular and Electrodiagnostic Medicine Loren M. Fishman, MD, B.Phil Robert A.Werner, MD, MS Scott J. Primack, DO Willam S. Pease, MD Ernest W. Johnson, MD Lawrence R. Robinson, MD 2005 COURSE F AANEM 52nd Annual Scientific Meeting Monterey, California AANEM Copyright © September 2005 American Association of Neuromuscular & Electrodiagnostic Medicine 421 First Avenue SW, Suite 300 East Rochester, MN 55902 PRINTED BY JOHNSON PRINTING COMPANY, INC. ii CROSSFIRE: Controversies in Neuromuscular and Electrodiagnostic Medicine Faculty Loren M. Fishman, MD, B.Phil Scott J. Primack, DO Assistant Clinical Professor Co-director Department of Physical Medicine and Rehabilitation Colorado Rehabilitation and Occupational Medicine Columbia College of Physicians and Surgeons Denver, Colorado New York City, New York Dr. Primack completed his residency at the Rehabilitation Institute of Dr. Fishman is a specialist in low back pain and sciatica, electrodiagnosis, Chicago in 1992. He then spent 6 months with Dr. Larry Mack at the functional assessment, and cognitive rehabilitation. Over the last 20 years, University of Washington. Dr. Mack, in conjunction with the Shoulder he has lectured frequently and contributed over 55 publications. His most and Elbow Service at the University of Washington, performed some of the recent work, Relief is in the Stretch: End Back Pain Through Yoga, and the original research utilizing musculoskeletal ultrasound in order to diagnose earlier book, Back Talk, both written with Carol Ardman, were published shoulder pathology. -

Sciatica and Chronic Pain
Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh 123 Sciatica and Chronic Pain Robert W. Baloh Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh, MD Department of Neurology University of California, Los Angeles Los Angeles, CA, USA ISBN 978-3-319-93903-2 ISBN 978-3-319-93904-9 (eBook) https://doi.org/10.1007/978-3-319-93904-9 Library of Congress Control Number: 2018952076 © Springer International Publishing AG, part of Springer Nature 2019 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. -

Surgery for Lumbar Radiculopathy/ Sciatica Final Evidence Report
Surgery for Lumbar Radiculopathy/ Sciatica Final evidence report April 13, 2018 Health Technology Assessment Program (HTA) Washington State Health Care Authority PO Box 42712 Olympia, WA 98504-2712 (360) 725-5126 www.hca.wa.gov/hta [email protected] Prepared by: RTI International–University of North Carolina Evidence-based Practice Center Research Triangle Park, NC 27709 www.rti.org This evidence report is based on research conducted by the RTI-UNC Evidence-based Practice Center through a contract between RTI International and the State of Washington Health Care Authority (HCA). The findings and conclusions in this document are those of the authors, who are responsible for its contents. The findings and conclusions do not represent the views of the Washington HCA and no statement in this report should be construed as an official position of Washington HCA. The information in this report is intended to help the State of Washington’s independent Health Technology Clinical Committee make well-informed coverage determinations. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information (i.e., in the context of available resources and circumstances presented by individual patients). This document is in the public domain and may be used and reprinted without permission except those copyrighted materials that are clearly noted in the document. Further reproduction of those copyrighted materials is prohibited without the specific permission of copyright holders. -

Lumbosacral Plexus Entrapment Syndrome. Part One: a Common Yet Little-Known Cause of Chronic Pelvic and Lower Extremity Pain
3-A Running head: ANAESTHESIA, PAIN & INTENSIVE CARE www.apicareonline.com ORIGINAL ARTICLE Lumbosacral plexus entrapment syndrome. Part one: A common yet little-known cause of chronic pelvic and lower extremity pain Kjetil Larsen, CES, George C. Chang Chien, D O2 ABSTRACT Corrective exercise specialist, Training & Rehabilitation, Oslo Lumbosacral plexus entrapment syndrome (LPES) is a little-known but common cause Norway of chronic lumbopelvic and lower extremity pain. The lumbar plexus, including the 2 Director of pain management, lumbosacral tunks emerge through the fibers of the psoas major, and the proximal Ventura County Medical Center, sciatic nerve beneath the piriformis muscles. Severe weakness of these muscles may Ventura, CA 93003, USA. lead to entrapment plexopathy, resulting in diffuse and non-specific pain patterns Correspondence: Kjetil Larsen, CES, Corrective throughout the lumbopelvic complex and lower extremities (LPLE), easily mimicking Exercise Specialist, Training & other diagnoses and is therefore likely to mislead the interpreting clinician. It is a Rehabilitation, Oslo Norway; pathology very similar to that of thoracic outlet syndrome, but for the lower body. This Kjetil@trainingandrehabilitation. two part manuscript series was written in an attempt to demonstrate the existence, com; pathophysiology, diagnostic protocol as well as interventional strategy for LPES, and Tel.: +47 975 45 192 its efficacy. Received: 23 November 2018, Reviewed & Accepted: 28 Key words: Pelvic girdle; Pain, Pelvic girdle; Lumbosacral plexus entrapment syndrome; February 2019 Piriformis syndrome; Nerve entrapment; Double-crush; Pain, Chronic; Fibromyalgia Citation: Larsen K, Chien GCC. Lumbosacral plexus entrapment syndrome. Part one: A common yet little-known cause of chronic pelvic and lower extremity pain. -

Neurological Syndromes of Brucellosis
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.51.8.1017 on 1 August 1988. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry 1988;51:1017-1021 Neurological syndromes of brucellosis M BAHEMUKA,* A RAHMAN SHEMENA, C P PANAYIOTOPOULOS, A K AL-ASKA, TAHIR OBEID, A K DAIF From the College ofMedicine, Department ofMedicine, Riyadh, Saudi Arabia SUMMARY Eleven patients with brucellosis presented with neurological features closely simulating transient ischaemic attacks, cerebral infarction, acute confusional state, motor neuron disease, progressive multisystem degeneration, polyradiculoneuropathy, neuralgic amyotrophy, sciatica and cauda equina syndrome. Most patients improved quickly after adequate antibiotic treatment but chronic cases responded poorly. These protean neurological manifestations of brucellosis indicate that the underlying pathological mechanisms are diverse. Brucellosis is a common infection in Saudi Arabia Case reports and still occurs in most developed countries.' 2 The nervous system may be affected diversely with com- Case 1 A 26 year old right handed Sudanese was admitted arising from the involvement of with a day's history of headache, vomiting, drowsiness, con- plex symptomatology fusion and fever. He had experienced intermittent bifrontal the meninges, cerebral vessels, central nervous system headache with no special features for 8 months. During this and cranial and peripheral nerves.2-5 period he had recurrent fever, was anorexic and had lost Protected by copyright. The purpose of this report is to document our ex- weight. Four months prior to admission he had been having perience of 11 cases whose presentation mimicked episodes of weakness in the right limbs associated with several neurological syndromes and diseases, most of difficulties in choosing the correct words although he could which improved with treatment. -

Performing Arts Safety Primer: Musicians And
Actsafe Performing Arts Safety Table of contents Primers Introduction...................................................4 This book is one in a series of Performing Arts Safety MSI symptoms...................................................5 Primers that also includes: MSIs common to musicians.............................8 Prevention....................................................18 • The Performing Arts Safety Primer Treatment.......................................................27 • Dancers and MSI When to seek medical assistance................30 Additional information...................................31 Actsafe would like to thank Dr. Robert Cannon and Dan Robin- son, PhD. CCPE RK for their contributions towards this Primer. Feedback Request: We’re always looking to improve the qual- ity of our outreach and publications. If you have suggestions for improving this publication, we’d love to hear from you. Feel free to contact us by email at [email protected]. Note: The material in this publication is intended as educational information only. This publication does not replace the Occupa- tional Health & Safety Regulation administered by WorkSafeBC. Employers and workers should always refer to the Regulation for specific requirements that apply to their activities. ©2010 Actsafe. All rights reserved. Actsafe encourages the copying, reproduction and distribution of this document to promote health & safety in the workplace, provided Actsafe is acknowledged. However, no part of this publication may be copied, reproduced or distributed for profit or other commercial enterprise, nor may any part be incorporated into any other publications, without written permission from Actsafe. First printing, December 2010. Printed in Canada. 3 Introduction MSI symptoms A Musculoskeletal injury (MSI) is any injury or If you develop an MSI, you may experience disorder of the muscles, bones, joints, tendons, symptoms that interfere with your ability to ligaments, nerves, blood vessels, or related soft perform at the level you are accustomed to, tissues. -

Use of Magnetic Resonance Neurography for Evaluating The
Original Article | Neuroimaging and Head & Neck eISSN 2005-8330 https://doi.org/10.3348/kjr.2019.0739 Korean J Radiol 2020;21(4):483-493 Use of Magnetic Resonance Neurography for Evaluating the Distribution and Patterns of Chronic Inflammatory Demyelinating Polyneuropathy Xiaoyun Su, PhD1, 2, Xiangquan Kong, PhD1, 2, Zuneng Lu, PhD3, Min Zhou, PhD1, 2, Jing Wang, PhD1, 2, Xiaoming Liu, MD1, 2, Xiangchuang Kong, MD1, 2, Huiting Zhang, PhD4, Chuansheng Zheng, PhD1, 2 1Department of Radiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; 2Hubei Province Key Laboratory of Molecular Imaging, Wuhan, China; 3Department of Neurology, Renming Hospital of Wuhan University, Wuhan, China; 4MR Scientific Marketing, Siemens Healthineers, Shanghai, China Objective: To evaluate the distribution and characteristics of peripheral nerve abnormalities in chronic inflammatory demyelinating polyneuropathy (CIDP) using magnetic resonance neurography (MRN) and to examine the diagnostic efficiency. Materials and Methods: Thirty-one CIDP patients and 21 controls underwent MR scans. Three-dimensional sampling perfections with application-optimized contrasts using different flip-angle evolutions and T1-/T2- weighted turbo spin- echo sequences were performed for neurography of the brachial and lumbosacral (LS) plexus and cauda equina, respectively. Clinical data and scores of the inflammatory Rasch-built overall disability scale (I-RODS) in CIDP were obtained. Results: The bilateral extracranial vagus (n = 11), trigeminal (n = 12), and intercostal nerves (n = 10) were hypertrophic. Plexus hypertrophies were observed in the brachial plexus of 19 patients (61.3%) and in the LS plexus of 25 patients (80.6%). Patterns of hypertrophy included uniform hypertrophy (17 [54.8%] brachial plexuses and 21 [67.7%] LS plexuses), and multifocal fusiform hypertrophy (2 [6.5%] brachial plexuses and 4 [12.9%] LS plexuses) was present. -

Free PDF Download
Eur opean Rev iew for Med ical and Pharmacol ogical Sci ences 2014; 18: 2766-2771 Peripheral neuropathy in obstetrics: efficacy and safety of α-lipoic acid supplementation M. COSTANTINO, C. GUARALDI 1, D. COSTANTINO 2, S. DE GRAZIA 3, V. UNFER 3 Chemistry and Pharmaceutical Technologies, University of Ferrara, Ferrara, Italy 1Obstetrics and Gynaecology Unit, Ospedale di Valdagno (VI), Italy 2Female Health Centre, Azienda USL, Ferrara, Italy 3A.G.UN.CO. Ostetric and Gynecological Center, Rome, Italy Abstract. – OBJECTIVE : Neuropathic pain more prone to the development of neuropathic during pregnancy is a common condition due to syndromes. First and foremost, the physical the physical changes and compression around changes caused by the enlargement of the uterus pregnancy and childbirth that make pregnant women more prone to develop several medical and the development of the foetus cause postural conditions such as carpal tunnel syndrome, sci - changes and nutation of the pelvic girdle that fa - atica, meralgia paraesthetica and other nerve en - cilitate the development of low back pain and en - trapment syndromes. Most of the treatments trapment neuropathies. The mutation of the usually performed to counteract neuropathic pelvic girdle is favoured during pregnancy also pain are contraindicated in pregnancy so that, by the presence of high concentrations of relaxin, the management of these highly invalidating conditions remains an issue in the clinical prac - which is produced from the tenth week of gesta - tice. We aimed to review the efficacy and safety tion and causes a laxity in the joints not only in of alpha lipoic acid supplementation in the treat - the pelvis, but also on a vertebral level, which ment of neuropathic pain. -

Evaluating the Patient with Suspected Radiculopathy
EVALUATINGEVALUATING THETHE PATIENTPATIENT WITHWITH SUSPECTEDSUSPECTED RADICULOPATHYRADICULOPATHY Timothy R. Dillingham, M.D., M.S Professor and Chair, Department of Physical Medicine and Rehabilitation The Medical College of Wisconsin. RadiculopathiesRadiculopathies PathophysiologicalPathophysiological processesprocesses affectingaffecting thethe nervenerve rootsroots VeryVery commoncommon reasonreason forfor EDXEDX referralreferral CAUSESCAUSES OFOF RADICULOPATHYRADICULOPATHY HNPHNP RadiculiitisRadiculiitis SpinalSpinal StenosisStenosis SpondylolisthesisSpondylolisthesis InfectionInfection TumorTumor FacetFacet SynovialSynovial CystCyst Diseases:Diseases: Diabetes,Diabetes, AIDPAIDP MUSCULOSKELETALMUSCULOSKELETAL DISORDERSDISORDERS :: UPPERUPPER LIMBLIMB ShoulderShoulder BursitisBursitis LateralLateral EpicondylitisEpicondylitis DequervainsDequervains TriggerTrigger fingerfinger FibrositisFibrositis FibromyalgiaFibromyalgia // regionalregional painpain syndromesyndrome NEUROLOGICALNEUROLOGICAL CONDITIONSCONDITIONS MIMICKINGMIMICKING CERVICALCERVICAL RADICULOPATHYRADICULOPATHY Entrapment/CompressionEntrapment/Compression neuropathiesneuropathies –– Median,Median, Radial,Radial, andand UlnarUlnar BrachialBrachial NeuritisNeuritis MultifocalMultifocal MotorMotor NeuropathyNeuropathy NeedNeed ExtensiveExtensive EDXEDX studystudy toto R/OR/O otherother conditionsconditions MUSCULOSKELETALMUSCULOSKELETAL DISORDERSDISORDERS :: LOWERLOWER LIMBLIMB HipHip arthritisarthritis TrochantericTrochanteric BursitisBursitis IlliotibialIlliotibial BandBand