Use of Magnetic Resonance Neurography for Evaluating The
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Neurophysiologic Testing and Monitoring
UnitedHealthcare® Commercial Medical Policy Neurophysiologic Testing and Monitoring Policy Number: 2021T0493Z Effective Date: January 1, 2021 Instructions for Use Table of Contents Page Community Plan Policy Coverage Rationale ........................................................................... 1 • Neurophysiologic Testing and Monitoring Applicable Codes .............................................................................. 2 Description of Services ..................................................................... 4 Medicare Advantage Coverage Summary Clinical Evidence ............................................................................... 6 • Neurophysiological Studies U.S. Food and Drug Administration ..............................................16 References .......................................................................................18 Policy History/Revision Information..............................................21 Instructions for Use .........................................................................22 Coverage Rationale Nerve Conduction Studies The following are proven and medically necessary: • Nerve conduction studies with or without late responses (e.g., F-wave and H-reflex tests) and neuromuscular junction testing when performed in conjunction with needle electromyography for any of the following known or suspected disorders: o Peripheral neuropathy/polyneuropathy (e.g., inherited, metabolic, traumatic, entrapment syndromes) o Plexopathy o Neuromuscular junction disorders (e.g., -
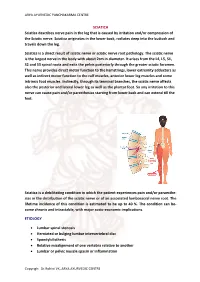
SCIATICA Sciatica Describes Nerve Pain in the Leg That Is Caused by Irritation And/Or Compression of the Sciatic Nerve
ARYA AYURVEDIC PANCHAKARMA CENTRE SCIATICA Sciatica describes nerve pain in the leg that is caused by irritation and/or compression of the Sciatic nerve. Sciatica originates in the lower back, radiates deep into the buttock and travels down the leg. Sciatica is a direct result of sciatic nerve or sciatic nerve root pathology. The sciatic nerve is the largest nerve in the body with about 2cm in diameter. It arises from the L4, L5, S1, S2 and S3 spinal roots and exits the pelvis posteriorly through the greater sciatic foramen. This nerve provides direct motor function to the hamstrings, lower extremity adductors as well as indirect motor function to the calf muscles, anterior lower leg muscles and some intrinsic foot muscles. Indirectly, through its terminal branches, the sciatic nerve affects also the posterior and lateral lower leg as well as the plantar foot. So any irritation to this nerve can cause pain and/or paresthesias starting from lower back and can extend till the feet. Sciatica is a debilitating condition in which the patient experiences pain and/or paraesthe- sias in the distribution of the sciatic nerve or of an associated lumbosacral nerve root. The lifetime incidence of this condition is estimated to be up to 40 %. The condition can be- come chronic and intractable, with major socio-economic implications. ETIOLOGY • Lumbar spinal stenosis • Herniated or bulging lumbar intervertebral disc • Spondylolisthesis • Relative misalignment of one vertebra relative to another • Lumbar or pelvic muscle spasm or inflammation Copyrigh: Dr.Rohini VK, ARYA AYURVEDIC CENTRE ARYA AYURVEDIC PANCHAKARMA CENTRE • Spinal or Paraspinal masses included malignancy, epidural haematoma or epidural abscess • Lumbar degenerative disc disease • Sacroiliac joint dysfunction EPIDEMIOLOGY GENDER: There appears to be no gender predominance. -

Radiculopathy Vs. Spinal Stenosis: Evocative Electrodiagnosis Identifies the Main Pain Generator
Functional Electromyography Loren M. Fishman · Allen N. Wilkins Functional Electromyography Provocative Maneuvers in Electrodiagnosis 123 Loren M. Fishman, MD Allen N. Wilkins, MD College of Physicians & Surgeons Manhattan Physical Medicine Columbia University and Rehabilitation New York, NY 10028, USA New York, NY 10013, USA [email protected] ISBN 978-1-60761-019-9 e-ISBN 978-1-60761-020-5 DOI 10.1007/978-1-60761-020-5 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010935087 © Springer Science+Business Media, LLC 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein. -

Piriformis Syndrome: the Literal “Pain in My Butt” Chelsea Smith, PTA
Piriformis Syndrome: the literal “pain in my butt” Chelsea Smith, PTA Aside from the monotony of day-to-day pains and annoyances, piriformis syndrome is the literal “pain in my butt” that may not go away with sending the kids to grandmas and often takes the form of sciatica. Many individuals with pain in the buttock that radiates down the leg are experiencing a form of sciatica caused by irritation of the spinal nerves in or near the lumbar spine (1). Other times though, the nerve irritation is not in the spine but further down the leg due to a pesky muscle called the piriformis, hence “piriformis syndrome”. The piriformis muscle is a flat, pyramidal-shaped muscle that originates from the front surface of the sacrum and the joint capsule of the sacroiliac joint (SI joint) and is located deep in the gluteal tissue (2). The piriformis travels through the greater sciatic foramen and attaches to the upper surface of the greater trochanter (or top of the hip bone) while the sciatic nerve runs under (and sometimes through) the piriformis muscle as it exits the pelvis. Due to this close proximity between the piriformis muscle and the sciatic nerve, if there is excessive tension (tightness), spasm, or inflammation of the piriformis muscle this can cause irritation to the sciatic nerve leading to symptoms of sciatica (pain down the leg) (1). Activities like sitting on hard surfaces, crouching down, walking or running for long distances, and climbing stairs can all increase symptoms (2) with the most common symptom being tenderness along the piriformis muscle (deep in the gluteal region) upon palpation. -

Piriformis Syndrome Is Overdiagnosed 11 Robert A
American Association of Neuromuscular & Electrodiagnostic Medicine AANEM CROSSFIRE: CONTROVERSIES IN NEUROMUSCULAR AND ELECTRODIAGNOSTIC MEDICINE Loren M. Fishman, MD, B.Phil Robert A.Werner, MD, MS Scott J. Primack, DO Willam S. Pease, MD Ernest W. Johnson, MD Lawrence R. Robinson, MD 2005 AANEM COURSE F AANEM 52ND Annual Scientific Meeting Monterey, California CROSSFIRE: Controversies in Neuromuscular and Electrodiagnostic Medicine Loren M. Fishman, MD, B.Phil Robert A.Werner, MD, MS Scott J. Primack, DO Willam S. Pease, MD Ernest W. Johnson, MD Lawrence R. Robinson, MD 2005 COURSE F AANEM 52nd Annual Scientific Meeting Monterey, California AANEM Copyright © September 2005 American Association of Neuromuscular & Electrodiagnostic Medicine 421 First Avenue SW, Suite 300 East Rochester, MN 55902 PRINTED BY JOHNSON PRINTING COMPANY, INC. ii CROSSFIRE: Controversies in Neuromuscular and Electrodiagnostic Medicine Faculty Loren M. Fishman, MD, B.Phil Scott J. Primack, DO Assistant Clinical Professor Co-director Department of Physical Medicine and Rehabilitation Colorado Rehabilitation and Occupational Medicine Columbia College of Physicians and Surgeons Denver, Colorado New York City, New York Dr. Primack completed his residency at the Rehabilitation Institute of Dr. Fishman is a specialist in low back pain and sciatica, electrodiagnosis, Chicago in 1992. He then spent 6 months with Dr. Larry Mack at the functional assessment, and cognitive rehabilitation. Over the last 20 years, University of Washington. Dr. Mack, in conjunction with the Shoulder he has lectured frequently and contributed over 55 publications. His most and Elbow Service at the University of Washington, performed some of the recent work, Relief is in the Stretch: End Back Pain Through Yoga, and the original research utilizing musculoskeletal ultrasound in order to diagnose earlier book, Back Talk, both written with Carol Ardman, were published shoulder pathology. -

Sciatica and Chronic Pain
Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh 123 Sciatica and Chronic Pain Robert W. Baloh Sciatica and Chronic Pain Past, Present and Future Robert W. Baloh, MD Department of Neurology University of California, Los Angeles Los Angeles, CA, USA ISBN 978-3-319-93903-2 ISBN 978-3-319-93904-9 (eBook) https://doi.org/10.1007/978-3-319-93904-9 Library of Congress Control Number: 2018952076 © Springer International Publishing AG, part of Springer Nature 2019 This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed. The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. The publisher, the authors, and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, express or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. -

Surgery for Lumbar Radiculopathy/ Sciatica Final Evidence Report
Surgery for Lumbar Radiculopathy/ Sciatica Final evidence report April 13, 2018 Health Technology Assessment Program (HTA) Washington State Health Care Authority PO Box 42712 Olympia, WA 98504-2712 (360) 725-5126 www.hca.wa.gov/hta [email protected] Prepared by: RTI International–University of North Carolina Evidence-based Practice Center Research Triangle Park, NC 27709 www.rti.org This evidence report is based on research conducted by the RTI-UNC Evidence-based Practice Center through a contract between RTI International and the State of Washington Health Care Authority (HCA). The findings and conclusions in this document are those of the authors, who are responsible for its contents. The findings and conclusions do not represent the views of the Washington HCA and no statement in this report should be construed as an official position of Washington HCA. The information in this report is intended to help the State of Washington’s independent Health Technology Clinical Committee make well-informed coverage determinations. This report is not intended to be a substitute for the application of clinical judgment. Anyone who makes decisions concerning the provision of clinical care should consider this report in the same way as any medical reference and in conjunction with all other pertinent information (i.e., in the context of available resources and circumstances presented by individual patients). This document is in the public domain and may be used and reprinted without permission except those copyrighted materials that are clearly noted in the document. Further reproduction of those copyrighted materials is prohibited without the specific permission of copyright holders. -

Lumbosacral Plexus Entrapment Syndrome. Part One: a Common Yet Little-Known Cause of Chronic Pelvic and Lower Extremity Pain
3-A Running head: ANAESTHESIA, PAIN & INTENSIVE CARE www.apicareonline.com ORIGINAL ARTICLE Lumbosacral plexus entrapment syndrome. Part one: A common yet little-known cause of chronic pelvic and lower extremity pain Kjetil Larsen, CES, George C. Chang Chien, D O2 ABSTRACT Corrective exercise specialist, Training & Rehabilitation, Oslo Lumbosacral plexus entrapment syndrome (LPES) is a little-known but common cause Norway of chronic lumbopelvic and lower extremity pain. The lumbar plexus, including the 2 Director of pain management, lumbosacral tunks emerge through the fibers of the psoas major, and the proximal Ventura County Medical Center, sciatic nerve beneath the piriformis muscles. Severe weakness of these muscles may Ventura, CA 93003, USA. lead to entrapment plexopathy, resulting in diffuse and non-specific pain patterns Correspondence: Kjetil Larsen, CES, Corrective throughout the lumbopelvic complex and lower extremities (LPLE), easily mimicking Exercise Specialist, Training & other diagnoses and is therefore likely to mislead the interpreting clinician. It is a Rehabilitation, Oslo Norway; pathology very similar to that of thoracic outlet syndrome, but for the lower body. This Kjetil@trainingandrehabilitation. two part manuscript series was written in an attempt to demonstrate the existence, com; pathophysiology, diagnostic protocol as well as interventional strategy for LPES, and Tel.: +47 975 45 192 its efficacy. Received: 23 November 2018, Reviewed & Accepted: 28 Key words: Pelvic girdle; Pain, Pelvic girdle; Lumbosacral plexus entrapment syndrome; February 2019 Piriformis syndrome; Nerve entrapment; Double-crush; Pain, Chronic; Fibromyalgia Citation: Larsen K, Chien GCC. Lumbosacral plexus entrapment syndrome. Part one: A common yet little-known cause of chronic pelvic and lower extremity pain. -

ANMC Specialty Clinic Services
Cardiology Dermatology Diabetes Endocrinology Ear, Nose and Throat (ENT) Gastroenterology General Medicine General Surgery HIV/Early Intervention Services Infectious Disease Liver Clinic Neurology Neurosurgery/Comprehensive Pain Management Oncology Ophthalmology Orthopedics Orthopedics – Back and Spine Podiatry Pulmonology Rheumatology Urology Cardiology • Cardiology • Adult transthoracic echocardiography • Ambulatory electrocardiology monitor interpretation • Cardioversion, electrical, elective • Central line placement and venous angiography • ECG interpretation, including signal average ECG • Infusion and management of Gp IIb/IIIa agents and thrombolytic agents and antithrombotic agents • Insertion and management of central venous catheters, pulmonary artery catheters, and arterial lines • Insertion and management of automatic implantable cardiac defibrillators • Insertion of permanent pacemaker, including single/dual chamber and biventricular • Interpretation of results of noninvasive testing relevant to arrhythmia diagnoses and treatment • Hemodynamic monitoring with balloon flotation devices • Non-invasive hemodynamic monitoring • Perform history and physical exam • Pericardiocentesis • Placement of temporary transvenous pacemaker • Pacemaker programming/reprogramming and interrogation • Stress echocardiography (exercise and pharmacologic stress) • Tilt table testing • Transcutaneous external pacemaker placement • Transthoracic 2D echocardiography, Doppler, and color flow Dermatology • Chemical face peels • Cryosurgery • Diagnosis -

What Is the Autonomic Nervous System?
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.suppl_3.iii31 on 21 August 2003. Downloaded from AUTONOMIC DISEASES: CLINICAL FEATURES AND LABORATORY EVALUATION *iii31 Christopher J Mathias J Neurol Neurosurg Psychiatry 2003;74(Suppl III):iii31–iii41 he autonomic nervous system has a craniosacral parasympathetic and a thoracolumbar sym- pathetic pathway (fig 1) and supplies every organ in the body. It influences localised organ Tfunction and also integrated processes that control vital functions such as arterial blood pres- sure and body temperature. There are specific neurotransmitters in each system that influence ganglionic and post-ganglionic function (fig 2). The symptoms and signs of autonomic disease cover a wide spectrum (table 1) that vary depending upon the aetiology (tables 2 and 3). In some they are localised (table 4). Autonomic dis- ease can result in underactivity or overactivity. Sympathetic adrenergic failure causes orthostatic (postural) hypotension and in the male ejaculatory failure, while sympathetic cholinergic failure results in anhidrosis; parasympathetic failure causes dilated pupils, a fixed heart rate, a sluggish urinary bladder, an atonic large bowel and, in the male, erectile failure. With autonomic hyperac- tivity, the reverse occurs. In some disorders, particularly in neurally mediated syncope, there may be a combination of effects, with bradycardia caused by parasympathetic activity and hypotension resulting from withdrawal of sympathetic activity. The history is of particular importance in the consideration and recognition of autonomic disease, and in separating dysfunction that may result from non-autonomic disorders. CLINICAL FEATURES c copyright. General aspects Autonomic disease may present at any age group; at birth in familial dysautonomia (Riley-Day syndrome), in teenage years in vasovagal syncope, and between the ages of 30–50 years in familial amyloid polyneuropathy (FAP). -

New Insights in Lumbosacral Plexopathy
New Insights in Lumbosacral Plexopathy Kerry H. Levin, MD Gérard Said, MD, FRCP P. James B. Dyck, MD Suraj A. Muley, MD Kurt A. Jaeckle, MD 2006 COURSE C AANEM 53rd Annual Meeting Washington, DC Copyright © October 2006 American Association of Neuromuscular & Electrodiagnostic Medicine 2621 Superior Drive NW Rochester, MN 55901 PRINTED BY JOHNSON PRINTING COMPANY, INC. C-ii New Insights in Lumbosacral Plexopathy Faculty Kerry H. Levin, MD P. James. B. Dyck, MD Vice-Chairman Associate Professor Department of Neurology Department of Neurology Head Mayo Clinic Section of Neuromuscular Disease/Electromyography Rochester, Minnesota Cleveland Clinic Dr. Dyck received his medical degree from the University of Minnesota Cleveland, Ohio School of Medicine, performed an internship at Virginia Mason Hospital Dr. Levin received his bachelor of arts degree and his medical degree from in Seattle, Washington, and a residency at Barnes Hospital and Washington Johns Hopkins University in Baltimore, Maryland. He then performed University in Saint Louis, Missouri. He then performed fellowships at a residency in internal medicine at the University of Chicago Hospitals, the Mayo Clinic in peripheral nerve and electromyography. He is cur- where he later became the chief resident in neurology. He is currently Vice- rently Associate Professor of Neurology at the Mayo Clinic. Dr. Dyck is chairman of the Department of Neurology and Head of the Section of a member of several professional societies, including the AANEM, the Neuromuscular Disease/Electromyography at Cleveland Clinic. Dr. Levin American Academy of Neurology, the Peripheral Nerve Society, and the is also a professor of medicine at the Cleveland Clinic College of Medicine American Neurological Association. -

A Clinical Study on the Utility of Nerve Biopsy in Peripheral Neuropathy
A CLINICAL STUDY ON THE UTILITY OF NERVE BIOPSY IN PERIPHERAL NEUROPATHY Thesis submitted for the partial fulfilment for the requirement of the degree of DM Neurology DR. JITESH GOEL DM NEUROLOGY RESIDENT 2014–2016 DEPARTMENT OF NEUROLOGY SREE CHITRA TIRUNAL INSTITUTE FOR MEDICAL SCIENCES AND TECHNOLOGY, TRIVANDRUM, KERALA 695011 i DECLARATION I, Dr Jitesh hereby declare that the thesis “A CLINICAL STUDY ON THE UTILITY OF NERVE BIOPSY IN PERIPHERAL NEUROPATHY” was undertaken by me under the guidance and supervision of Dr MD Nair, Senior Professor and Head of Department, Department of Neurology at the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram. Dr.Jitesh Goel Thiruvananthapuram Senior Resident Date: Dept. of Neurology SCTIMST Thiruvananthapuram ii CERTIFICATE This is to certify that the thesis titled “A CLINICAL STUDY ON THE UTILITY OF NERVE BIOPSY IN PERIPHERAL NEUROPATHY”, is the bonafide work of Dr Jitesh Goel, Senior Resident, DM Neurology and has been done under my direct guidance and supervision at the Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram. He has shown keen interest in the research project and actively participated in all its phases. Thiruvananthapuram Dr MD Nair (Guide) Date: Senior Professor and Head of Department Department of Neurology, SCTIMST. Thiruvananthapuram iii CONTENTS Sl. No. Title Page No. 1 Introduction 1 2 Review of Literature 3 3 Aim of The Study 32 4 Materials And Methods 32 5 Results 34 6 Discussion 62 7 Conclusion 72 8 References 75 9 Annexures 84 IEC Approval Proforma iv INTRODUCTION Peripheral neuropathy is among the common disorders in patients attending neuromuscular clinic.