Ephemeroptera: Baetiscidae)
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ohio EPA Macroinvertebrate Taxonomic Level December 2019 1 Table 1. Current Taxonomic Keys and the Level of Taxonomy Routinely U
Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Table 1. Current taxonomic keys and the level of taxonomy routinely used by the Ohio EPA in streams and rivers for various macroinvertebrate taxonomic classifications. Genera that are reasonably considered to be monotypic in Ohio are also listed. Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Species Pennak 1989, Thorp & Rogers 2016 Porifera If no gemmules are present identify to family (Spongillidae). Genus Thorp & Rogers 2016 Cnidaria monotypic genera: Cordylophora caspia and Craspedacusta sowerbii Platyhelminthes Class (Turbellaria) Thorp & Rogers 2016 Nemertea Phylum (Nemertea) Thorp & Rogers 2016 Phylum (Nematomorpha) Thorp & Rogers 2016 Nematomorpha Paragordius varius monotypic genus Thorp & Rogers 2016 Genus Thorp & Rogers 2016 Ectoprocta monotypic genera: Cristatella mucedo, Hyalinella punctata, Lophopodella carteri, Paludicella articulata, Pectinatella magnifica, Pottsiella erecta Entoprocta Urnatella gracilis monotypic genus Thorp & Rogers 2016 Polychaeta Class (Polychaeta) Thorp & Rogers 2016 Annelida Oligochaeta Subclass (Oligochaeta) Thorp & Rogers 2016 Hirudinida Species Klemm 1982, Klemm et al. 2015 Anostraca Species Thorp & Rogers 2016 Species (Lynceus Laevicaudata Thorp & Rogers 2016 brachyurus) Spinicaudata Genus Thorp & Rogers 2016 Williams 1972, Thorp & Rogers Isopoda Genus 2016 Holsinger 1972, Thorp & Rogers Amphipoda Genus 2016 Gammaridae: Gammarus Species Holsinger 1972 Crustacea monotypic genera: Apocorophium lacustre, Echinogammarus ischnus, Synurella dentata Species (Taphromysis Mysida Thorp & Rogers 2016 louisianae) Crocker & Barr 1968; Jezerinac 1993, 1995; Jezerinac & Thoma 1984; Taylor 2000; Thoma et al. Cambaridae Species 2005; Thoma & Stocker 2009; Crandall & De Grave 2017; Glon et al. 2018 Species (Palaemon Pennak 1989, Palaemonidae kadiakensis) Thorp & Rogers 2016 1 Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Informal grouping of the Arachnida Hydrachnidia Smith 2001 water mites Genus Morse et al. -

TB142: Mayflies of Maine: an Annotated Faunal List
The University of Maine DigitalCommons@UMaine Technical Bulletins Maine Agricultural and Forest Experiment Station 4-1-1991 TB142: Mayflies of aine:M An Annotated Faunal List Steven K. Burian K. Elizabeth Gibbs Follow this and additional works at: https://digitalcommons.library.umaine.edu/aes_techbulletin Part of the Entomology Commons Recommended Citation Burian, S.K., and K.E. Gibbs. 1991. Mayflies of Maine: An annotated faunal list. Maine Agricultural Experiment Station Technical Bulletin 142. This Article is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Technical Bulletins by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. ISSN 0734-9556 Mayflies of Maine: An Annotated Faunal List Steven K. Burian and K. Elizabeth Gibbs Technical Bulletin 142 April 1991 MAINE AGRICULTURAL EXPERIMENT STATION Mayflies of Maine: An Annotated Faunal List Steven K. Burian Assistant Professor Department of Biology, Southern Connecticut State University New Haven, CT 06515 and K. Elizabeth Gibbs Associate Professor Department of Entomology University of Maine Orono, Maine 04469 ACKNOWLEDGEMENTS Financial support for this project was provided by the State of Maine Departments of Environmental Protection, and Inland Fisheries and Wildlife; a University of Maine New England, Atlantic Provinces, and Quebec Fellow ship to S. K. Burian; and the Maine Agricultural Experiment Station. Dr. William L. Peters and Jan Peters, Florida A & M University, pro vided support and advice throughout the project and we especially appreci ated the opportunity for S.K. Burian to work in their laboratory and stay in their home in Tallahassee, Florida. -

Appendix 11.3
APPENDIX 11.3: Macro-invertebrate diversity in Maine and northeastern U.S., by family. Maine data are from MABP database, from multiple sources. New England data (6 states, excluding New York) are from a compilation of data by Chandler and Loose (2001) that includes information for all states, but appears to focus on Massachusetts. Species totals include taxa that are identified only to genus level (i.e. genera without any species indicated). Appendices 15 Appendix 11.3 New England Taxa MABP records records Phylum Class Order Family # Genera # Spp %"Orphan" Genera *** # Genera # Spp Annelida Polychaeta Sabellida Sabellidae 00 00 Annelida Polychaeta Sabellida Aeolosomatidae 22 100 -- -- Annelida Clitellata Lumbiculida Lubriculidae 44 75 2 3 Annelida Clitellata Enchytraeida Enchytraeidae ? ? Annelida Clitellata Haplotoxida Naididae 14 35 71018 Annelida Clitellata Haplotoxida Tubificidae 612 17 5 10 Annelida Clitellata Lumbricida Glossoscolecidae -- -- Annelida Clitellata Branchiobdellida Bdellodrilidae 11 011 Annelida Clitellata Branchiobdellida Branchiobdellidae 11 011 Annelida Clitellata Branchiobdellida Cambarincolidae 24 024 Annelida Clitellata Rhynchobdellida Glossiphoniidae 77 0616 Annelida Clitellata Rhynchobdellida Piscicolidae 44 044 Annelida Clitellata Arhynchobdellida Hirudinididae 22 024 Annelida Clitellata Arhynchobdellida Erpobdellidae 45 048 Arthropoda Malacostraca Isopoda Asellidae 24 50 2 4 Arthropoda Malacostraca Amphipoda Gammaridae 11 014 Arthropoda Malacostraca Amphipoda Crangonyctidae 22 027 Arthropoda Malacostraca -
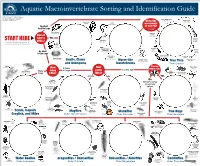
Aquatic Macroinvertebrate Sorting and Identification Guide
Aquatic Macroinvertebrate Sorting and Identification Guide Images courtesy of Troutnut.com, University of Wisconson Extension – ERC Natural Resources Education, University of South Florida College of Education – Florida Center for Instructional Biting Midge Larvae Technology, and Magnus Manske. Ceratopogonidae Midge Larvae Pouch Snails Worm-like Chironomidae Physidae Leeches Order Hirudinea Invertebrate No Shell or True Fly? And No Legs Use images to identify. Zebra Mussels Order Pelecypoda Soldier Fly Larvae Does it Stratiomyidae have a Yes Black Fly Planarians Larvae START HERE Simuliidae Please note that the illustrations are for SHELL? Order Turbellaria Orb reference only and are not true to scale. Snails Order Gastropoda Aquatic Worms Mosquito (Earthworm) Larvae Horse Fly Order Oligochaeta Culicidae Larvae No Shell Tabanidae It Has Legs Aquatic Worms Crane Fly Larvae (Tubifex) Tipulidae Fingernail Clams Order Oligochaeta Order Pelecypoda Horsehair Worms Shore Fly Larvae Snails, Clams Worm-like Order Nematomorpha True Flies Ephydridae and Unknowns Invertebrates Order Diptera Crayfish Piercing Order Decapoda How Water Striders How 3 Pairs MOUTH Part? Gerridae Scuds of Legs 1 or No Tail (Side Swimmer) Many many many Pigmy Order Amphipoda Legs Backswimmers Giant Water Bugs LEGS? TAILS? Pleidae Belostomatidae BEAK 3 Tails 2 Tails Yes Flathead No Mayfly Larvae Identify using Heptageniidae image key Small Squaregill below. Mayfly Larvae Caenidae Giant Stonefly Larvae Pteronarcyidae Isopods (Sowbug) Order Isopoda Spiny Crawler Water Small Water -

Appendix 2 Macroinvertebrates 011311
APPENDIX 2 Macroinvertebrates Abstract This appendix reviews the available evidence concerning the potential effects of the activities associated with the Spruce No. 1 Mine on the macroinvertebrate community of receiving streams and presents survey results from streams directly affected by the Spruce No. 1 Mine, including Oldhouse Branch and Pigeonroost Branch, and comparative data from adjacent mined streams impacted by the Dal-Tex operation, including Beech Creek, Left Fork Beech Creek, Rockhouse Creek, and Spruce Fork (Figure A2.1.). Figure A2.1. Map of Spruce No. 1 Mine area and adjacent Dal-Tex operation. EPA conducted three different analyses. First, EPA compared benthic macroinvertebrate community composition from Pigeonroost Branch and Oldhouse Branch to benthic macroinvertebrate community composition from streams that have been impacted by Mingo Logan's Dal-Tex operation. Second, EPA used an observed/expected approach to estimate and quantify taxonomic changes following mining disturbance. Third, EPA compared WVSCI scores in Pigeonroost Branch and Oldhouse Branch with streams impacted by the Dal-Tex operation. The results showed that some naturally occurring taxa will be locally extirpated in the receiving streams and will likely be replaced by pollution-tolerant taxa if mining and filling proceed. These results are supported by the State of West Virginia’s multimetric index (WVSCI), which also indicates that the magnitude and extent of degradation will increase following mining. The appendix also 1 includes a discussion of appropriate invertebrate metrics and data collection and analysis methods and explains why EPA focuses on changes to sensitive taxa and community composition. A2.1. Introduction Macroinvertebrates are good indicators of ecosystem health, and are used by West Virginia and other states in the Mid-Atlantic region and across the U.S. -

Ephemeroptera: Baetiscidae)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Crossref LIFE HISTORY AND ECOLOGY OF BAETISCA BAJKOVI NEAVE, IN BEECH FORK OF TWELVEPOLE CREEK, WAYNE COUNTY, WEST VIRGINIA (EPHEMEROPTERA: BAETISCIDAE) BY DWIGHT L. CHAFFEE AND DONALD C. TARTER The primary objective of this study was to investigate the life history and ecology of the mayfly Baetisca bajkovi Neave in Beech Fork of Twelvepole Creek, Wayne County, West Virginia. Several investigators, including Traver (1931), McDunnough (1932), Berner (1940, 1955), Edmunds (1960), Schneider and Berner (1963), Lehm- kuh! (1972), Pescador and Peters (1971, 1974), Morris (1976), and Tarter and Kirchner (1978), have reported taxonomical and ecologi- cal information on the genus Baetisca in North America. TAXONOMY AND DISTRIBUTION The family Baetiscidae is endemic in North America and contains only the genus Baetisca. The genus was established by Walsh (1862). Neave (1934) and Daggy (1945) described the nymph and imago, respectively, of B. bajkovi. The Nearctic distribution of B. bajkovi includes Alberta, Manitoba, and Saskatchewan in Canada (Neave, 1934 and Lehmkuhl, 1972), and Illinois, Indiana, Minnesota (Burks, 1953), Wisconsin (Hilsenhoff, 1970), and West Virginia in the Unit- ed States. Chaffee (1978) recorded it from 17 counties in West Virginia. The B. bajkovi nymphs were found mostly in a stream substrate that was predominantly small stones and sand, and with leaf litter or other plant material present. They were found only in the riffles, and were most frequently collected from water 10 to 26 cm in depth. Part of a thesis submitted by the first author to the Graduate School, Marshall University, in partial fulfillment of the requirements for the degree of Master of Science, December 1978. -

A Confirmed Record of the Ephemeroptera Genus Baetisca from West of the Continental Divide and an Annotated List of the Mayflies of the Humboldt River, Nevada
Western North American Naturalist Volume 60 Number 4 Article 15 10-31-2000 A confirmed ecorr d of the Ephemeroptera genus Baetisca from west of the Continental Divide and an annotated list of the mayflies of the Humboldt River, Nevada Richard W. Baumann Brigham Young University Boris C. Kondratieff Colorado State University, Fort Collins Follow this and additional works at: https://scholarsarchive.byu.edu/wnan Recommended Citation Baumann, Richard W. and Kondratieff, Boris C. (2000) "A confirmed ecorr d of the Ephemeroptera genus Baetisca from west of the Continental Divide and an annotated list of the mayflies of the Humboldt River, Nevada," Western North American Naturalist: Vol. 60 : No. 4 , Article 15. Available at: https://scholarsarchive.byu.edu/wnan/vol60/iss4/15 This Note is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Western North American Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Western North American Naturalist 60(4), © 2000, pp. 459–461 A CONFIRMED RECORD OF THE EPHEMEROPTERA GENUS BAETISCA FROM WEST OF THE CONTINENTAL DIVIDE AND AN ANNOTATED LIST OF THE MAYFLIES OF THE HUMBOLDT RIVER, NEVADA Richard W. Baumann1 and Boris C. Kondratieff2 ABSTRACT.—The mayfly Baetisca lacustris is reported from the Humboldt River, Nevada, based on 3 relatively mature nymphs collected in 1997 and 2000. An annotated list of the mayfly fauna of the Humboldt River is given, which includes the section from Elko to Winnemucca. Key words: mayflies, Ephemeroptera, Baetisca, Humboldt River, Nevada. -

Check List 2007: 3(1) ISSN: 1809-127X
Check List 2007: 3(1) ISSN: 1809-127X NOTES ON GEOGRAPHIC DISTRIBUTION Insecta, Ephemeroptera: Transcontinental (Metretopodidae); Ephoron album (Say) range extensions in western North America. (Polymitarcyidae); and Siphlonurus alternatus (Say) (northern) (Siphlonuridae). In addition to W. P. McCafferty1 these transcontinental species, there are a few M. D. Meyer2 others that are disjunct East and West species that are absent to a considerable extent in central 1Department of Entomology, Purdue University. regions of the continent. West Lafayette, Indiana, USA 47907. E-mail: [email protected] Based on our recent studies of mayflies from the 2Department of Biology, Chemistry, and west coast states of California, Oregon, and Environmental Science. Christopher Newport Washington, and the western intermountain USA University, 1 University Place, Newport, Virginia states (esp. Idaho), we are able to establish eight USA 23606. additional North American species with continuous transcontinental distribution patterns. In keeping Among the 631 valid species of Ephemeroptera with the trend among families shown above, six of (mayflies) that are presently known from North these species are in the family Baetidae, and one is America (McCafferty 2007), relatively few have in the family Caenidae. We also demonstrate this been known as having more or less continuous distribution pattern in the family Pseudironidae (non disjunct) transcontinental distribution patterns for the first time. New western state records that from the east coastal provinces of Canada and or substantiate the transcontinental patterns are given east coastal states of the USA to the west coastal for each of the species treated below, followed by provinces of Canada or the west coastal states of pertinent commentary regarding their distribution. -

Qt2cd0m6cp Nosplash 6A8244
International Advances in the Ecology, Zoogeography, and Systematics of Mayflies and Stoneflies Edited by F. R. Hauer, J. A. Stanford and, R. L. Newell International Advances in the Ecology, Zoogeography, and Systematics of Mayflies and Stoneflies Edited by F. R. Hauer, J. A. Stanford, and R. L. Newell University of California Press Berkeley Los Angeles London University of California Press, one of the most distinguished university presses in the United States, enriches lives around the world by advancing scholarship in the humanities, social sciences, and natural sciences. Its activities are supported by the UC Press Foundation and by philanthropic contributions from individuals and institutions. For more information, visit www.ucpress.edu. University of California Publications in Entomology, Volume 128 Editorial Board: Rosemary Gillespie, Penny Gullan, Bradford A. Hawkins, John Heraty, Lynn S. Kimsey, Serguei V. Triapitsyn, Philip S. Ward, Kipling Will University of California Press Berkeley and Los Angeles, California University of California Press, Ltd. London, England © 2008 by The Regents of the University of California Printed in the United States of America Library of Congress Cataloging-in-Publication Data International Conference on Ephemeroptera (11th : 2004 : Flathead Lake Biological Station, The University of Montana) International advances in the ecology, zoogeography, and systematics of mayflies and stoneflies / edited by F.R. Hauer, J.A. Stanford, and R.L. Newell. p. cm. – (University of California publications in entomology ; 128) "Triennial Joint Meeting of the XI International Conference on Ephemeroptera and XV International Symposium on Plecoptera held August 22-29, 2004 at Flathead Lake Biological Station, The University of Montana, USA." – Pref. Includes bibliographical references and index. -

Mayfly Families in North America
Module 2. Mayflies Mayfly Families in North America  *Baetidae – Baetis, very fast swimmer fusiform in shape  *Heptageniidae – Stenonema, common flat mayfly  Metretopodidae – Siphloplecton  *Oligoneuriidae – Isonychia, common filter-feeder  *Siphlonuridae – Ameletus  *Leptophlebiidae – Paraleptophlebia, small streams  Behnigiidae – Dolania, infrequent in coastal plain rivers  *Ephemeridae – Hexagenia, common burrower As of Brigham et. al. 1982 or Voshell 2003, *common families in piedmont and mountain streams Part of "Introduction to Taxonomy & Ecology of EPT" (NCSU Workshop Series, Funded by NCDENR & EPA 319) 1 Module 2. Mayflies Mayflies (continued)  Palingeniidae – Pentagenia  Polymitarcyidae – Ephoron  Potamanthidae – Potamanthus  *Ephemerellidae – Ephemerella, mostly mountains  Tricorythidae – Tricorythodes  *Caenidae – Caenis, operculate gills  Neoephemeridae – Neoephemera  Baetiscidae – Baetisca, enlarged pronotem As of Brigham et. al. 1982 or Voshell 2003, *common families in piedmont and mountain streams Basic Mayfly Morphology Mayfly Gills Part of "Introduction to Taxonomy & Ecology of EPT" (NCSU Workshop Series, Funded by NCDENR & EPA 319) 2 Module 2. Mayflies Dichotomous key to mayfly families Question, numbers from 1. Thoracic notum enlarged Brigham and Brigham Y N Baetiscidae Family name 2 Mandibular Tusks Present Y N Behningiidae 7. Operculate gills present N 9. Gills absent on 1 (2,3) Y 10. Gills present on 3 or 4 to 7 Y N YN Potamanthidae 8. Gills fused 11 Gills forked or clusters of Ephemerellidae Tricorythidae filaments Y Palingeniidae N Y N Neoephemeridae Caenidae Leptophlebiidae 12. Forelegs with filtering hairs Ephemeridae Y N Oligoneuriidae Polymitarcyidae 13. head and body flattened 14. Claws of foreleg bifid 15 Antennae short N YYY Heptageniidae Metrotopodidae Siphlonuridae Baetidae Key to Mayfly Families (continued) 1. -

The Mayfly Family Baetiscidae (Ephemeroptera). Part I Abstract Introduction
rrc,m: Ar-v/~f·lC·F~-f1 ;;\: ~~;:::; ...':-~--_,!r'·i·:_: :-· 1. :- \ ·:J;c;L()G'f ~·~- ~·., _·· ,, ____ ,_,>-:.. ;-·;.-.;JViarshall {:-·:~-.:nl! ~11 ?ut,;'.shir:g ~::o: ;.:1c.;i· !.:t;0n, 196:0) THE MAYFLY FAMILY BAETISCIDAE (EPHEMEROPTERA). PART I Lewis Berner and Manuel L. Pescador* Department of Zoology, University of Florida Gainesville, Florida 32611 ABSTRACT The history of the taxonomy and phylogeny of Baetisca~ the single genus in the family Baetiscidae, is reviewed beginning with the description of Prosopistoma as a crustacean. The relationship of the Prosopistomatidae to the Baetiscidae is treated as well as the concurrence of the authors with Edmunds' conclusion that the two families are derived from a common ancestor but parallel evolution must explain some of their similarities. There is a summary of presently recognized species and characteristics by which nymphs are differentiated. A brief treatment of the ecology and life history of various species is included. INTRODUCTION If Latreille had had nymphal specimens of Baetisca in 1833, would he have been as puzzled by them as he was by Prosopistoma when he described these curious nymphs as a new genus of crustacean? Certainly, Baetisca nymphs are sufficiently different from other Ephemeroptera to cause one to conclude that he might have done so. The superficial similarities of the mesonotal shields and the gills were enough to lead early entomologists to the misconception that the two are very closely related. Only in recent years, through * Laboratory of Aquatic Entomology, Florida A&M University, Tallahassee, Florida 32307 511 512 LEWIS BERNER AND MANUEL L. PESCADOR modern phylogenetic studies of all stages, has it been recognized that there are significant differences between them. -

SISTER RELATIONSHIP of the NEOEPHEMERIDAE and CAENIDAE (EPHEMEROPTERA: PANNOTA)L,2 T.-Q
52 ENTOMOLOGICAL NEWS SISTER RELATIONSHIP OF THE NEOEPHEMERIDAE AND CAENIDAE (EPHEMEROPTERA: PANNOTA)l,2 T.-Q. Wang3, W. P. McCafferty3, Y. J. Bae4 ABSTRACT: A consistent structural characteristic has been found to be unique to the mayfly fami lies Neoephemeridae and Caenidae. It is termed a sutural ommation and is present on the adult mesonotum. The structure is described and illustrated. Its unique presence supports the hypothesis of a sister relationship of Neoephemeridae and Caenidae among the pannote mayflies, a cladistic arrangement that has been somewhat debatable in the past. The Baetiscidae and Prosopistomatidae, which previously have been hypothesized to be closely related to either Neoephemeridae or Caenidae by various authors, are considered to constitute an aberrant clade of mayflies with still dubious relationships within the Ephemeroptera. Neoephemerid mayflies, the "large squaregills" (McCafferty 1981), were considered among the burrowing mayflies in the first half of this century. For example, Traver (1935) considered them as one of the subfamilies of Ephemeridae (=families ofEphemeroidea), and Ulmer (1939) considered them in the family Potamanthidae. This association was based on the common pos session of basally arched MP2 and CuA veins in the forewings. Edmunds and Traver (1954) placed the neoephemerids with the Caenidae, the "small squaregills" (McCafferty 1981), in a separate superfamily Caenoidea. This association was based on larval morphology, in particular the similar gill struc ture. Since that time, all workers, with the exception of Demoulin ( 1958), have grouped Neoephemeridae with Caenidae rather than Ephemeroidea. McCafferty and Edmunds (1979) placed the Caenoidea among other mayflies that possess the apomorphic characteristic of more or less fused developing wingpads and are known as the pannote mayflies.