Ephemeroptera: Baetiscidae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ohio EPA Macroinvertebrate Taxonomic Level December 2019 1 Table 1. Current Taxonomic Keys and the Level of Taxonomy Routinely U
Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Table 1. Current taxonomic keys and the level of taxonomy routinely used by the Ohio EPA in streams and rivers for various macroinvertebrate taxonomic classifications. Genera that are reasonably considered to be monotypic in Ohio are also listed. Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Species Pennak 1989, Thorp & Rogers 2016 Porifera If no gemmules are present identify to family (Spongillidae). Genus Thorp & Rogers 2016 Cnidaria monotypic genera: Cordylophora caspia and Craspedacusta sowerbii Platyhelminthes Class (Turbellaria) Thorp & Rogers 2016 Nemertea Phylum (Nemertea) Thorp & Rogers 2016 Phylum (Nematomorpha) Thorp & Rogers 2016 Nematomorpha Paragordius varius monotypic genus Thorp & Rogers 2016 Genus Thorp & Rogers 2016 Ectoprocta monotypic genera: Cristatella mucedo, Hyalinella punctata, Lophopodella carteri, Paludicella articulata, Pectinatella magnifica, Pottsiella erecta Entoprocta Urnatella gracilis monotypic genus Thorp & Rogers 2016 Polychaeta Class (Polychaeta) Thorp & Rogers 2016 Annelida Oligochaeta Subclass (Oligochaeta) Thorp & Rogers 2016 Hirudinida Species Klemm 1982, Klemm et al. 2015 Anostraca Species Thorp & Rogers 2016 Species (Lynceus Laevicaudata Thorp & Rogers 2016 brachyurus) Spinicaudata Genus Thorp & Rogers 2016 Williams 1972, Thorp & Rogers Isopoda Genus 2016 Holsinger 1972, Thorp & Rogers Amphipoda Genus 2016 Gammaridae: Gammarus Species Holsinger 1972 Crustacea monotypic genera: Apocorophium lacustre, Echinogammarus ischnus, Synurella dentata Species (Taphromysis Mysida Thorp & Rogers 2016 louisianae) Crocker & Barr 1968; Jezerinac 1993, 1995; Jezerinac & Thoma 1984; Taylor 2000; Thoma et al. Cambaridae Species 2005; Thoma & Stocker 2009; Crandall & De Grave 2017; Glon et al. 2018 Species (Palaemon Pennak 1989, Palaemonidae kadiakensis) Thorp & Rogers 2016 1 Ohio EPA Macroinvertebrate Taxonomic Level December 2019 Taxon Subtaxon Taxonomic Level Taxonomic Key(ies) Informal grouping of the Arachnida Hydrachnidia Smith 2001 water mites Genus Morse et al. -

TB142: Mayflies of Maine: an Annotated Faunal List
The University of Maine DigitalCommons@UMaine Technical Bulletins Maine Agricultural and Forest Experiment Station 4-1-1991 TB142: Mayflies of aine:M An Annotated Faunal List Steven K. Burian K. Elizabeth Gibbs Follow this and additional works at: https://digitalcommons.library.umaine.edu/aes_techbulletin Part of the Entomology Commons Recommended Citation Burian, S.K., and K.E. Gibbs. 1991. Mayflies of Maine: An annotated faunal list. Maine Agricultural Experiment Station Technical Bulletin 142. This Article is brought to you for free and open access by DigitalCommons@UMaine. It has been accepted for inclusion in Technical Bulletins by an authorized administrator of DigitalCommons@UMaine. For more information, please contact [email protected]. ISSN 0734-9556 Mayflies of Maine: An Annotated Faunal List Steven K. Burian and K. Elizabeth Gibbs Technical Bulletin 142 April 1991 MAINE AGRICULTURAL EXPERIMENT STATION Mayflies of Maine: An Annotated Faunal List Steven K. Burian Assistant Professor Department of Biology, Southern Connecticut State University New Haven, CT 06515 and K. Elizabeth Gibbs Associate Professor Department of Entomology University of Maine Orono, Maine 04469 ACKNOWLEDGEMENTS Financial support for this project was provided by the State of Maine Departments of Environmental Protection, and Inland Fisheries and Wildlife; a University of Maine New England, Atlantic Provinces, and Quebec Fellow ship to S. K. Burian; and the Maine Agricultural Experiment Station. Dr. William L. Peters and Jan Peters, Florida A & M University, pro vided support and advice throughout the project and we especially appreci ated the opportunity for S.K. Burian to work in their laboratory and stay in their home in Tallahassee, Florida. -

100 Characters
40 Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation: Final Report Leon C. Hinz Jr. and James N. Zahniser Illinois Natural History Survey Prairie Research Institute University of Illinois 30 April 2015 INHS Technical Report 2015 (31) Prepared for: Illinois Department of Natural Resources State Wildlife Grant Program (Project Number T-88-R-001) Unrestricted: for immediate online release. Prairie Research Institute, University of Illinois at Urbana Champaign Brian D. Anderson, Interim Executive Director Illinois Natural History Survey Geoffrey A. Levin, Acting Director 1816 South Oak Street Champaign, IL 61820 217-333-6830 Final Report Project Title: Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation. Project Number: T-88-R-001 Contractor information: University of Illinois at Urbana/Champaign Institute of Natural Resource Sustainability Illinois Natural History Survey 1816 South Oak Street Champaign, IL 61820 Project Period: 1 October 2013—31 September 2014 Principle Investigator: Leon C. Hinz Jr., Ph.D. Stream Ecologist Illinois Natural History Survey One Natural Resources Way, Springfield, IL 62702-1271 217-785-8297 [email protected] Prepared by: Leon C. Hinz Jr. & James N. Zahniser Goals/ Objectives: (1) Review all SGNC listing criteria for currently listed non-mollusk invertebrate species using criteria in Illinois Wildlife Action Plan, (2) Assess current status of species populations, (3) Review criteria for additional species for potential listing as SGNC, (4) Assess stressors to species previously reviewed, (5) Complete draft updates and revisions of IWAP Appendix I and Appendix II for non-mollusk invertebrates. T-88 Final Report Project Title: Review and Update of Non-mollusk Invertebrate Species in Greatest Need of Conservation. -

Appendix 11.3
APPENDIX 11.3: Macro-invertebrate diversity in Maine and northeastern U.S., by family. Maine data are from MABP database, from multiple sources. New England data (6 states, excluding New York) are from a compilation of data by Chandler and Loose (2001) that includes information for all states, but appears to focus on Massachusetts. Species totals include taxa that are identified only to genus level (i.e. genera without any species indicated). Appendices 15 Appendix 11.3 New England Taxa MABP records records Phylum Class Order Family # Genera # Spp %"Orphan" Genera *** # Genera # Spp Annelida Polychaeta Sabellida Sabellidae 00 00 Annelida Polychaeta Sabellida Aeolosomatidae 22 100 -- -- Annelida Clitellata Lumbiculida Lubriculidae 44 75 2 3 Annelida Clitellata Enchytraeida Enchytraeidae ? ? Annelida Clitellata Haplotoxida Naididae 14 35 71018 Annelida Clitellata Haplotoxida Tubificidae 612 17 5 10 Annelida Clitellata Lumbricida Glossoscolecidae -- -- Annelida Clitellata Branchiobdellida Bdellodrilidae 11 011 Annelida Clitellata Branchiobdellida Branchiobdellidae 11 011 Annelida Clitellata Branchiobdellida Cambarincolidae 24 024 Annelida Clitellata Rhynchobdellida Glossiphoniidae 77 0616 Annelida Clitellata Rhynchobdellida Piscicolidae 44 044 Annelida Clitellata Arhynchobdellida Hirudinididae 22 024 Annelida Clitellata Arhynchobdellida Erpobdellidae 45 048 Arthropoda Malacostraca Isopoda Asellidae 24 50 2 4 Arthropoda Malacostraca Amphipoda Gammaridae 11 014 Arthropoda Malacostraca Amphipoda Crangonyctidae 22 027 Arthropoda Malacostraca -

Microsoft Outlook
Joey Steil From: Leslie Jordan <[email protected]> Sent: Tuesday, September 25, 2018 1:13 PM To: Angela Ruberto Subject: Potential Environmental Beneficial Users of Surface Water in Your GSA Attachments: Paso Basin - County of San Luis Obispo Groundwater Sustainabilit_detail.xls; Field_Descriptions.xlsx; Freshwater_Species_Data_Sources.xls; FW_Paper_PLOSONE.pdf; FW_Paper_PLOSONE_S1.pdf; FW_Paper_PLOSONE_S2.pdf; FW_Paper_PLOSONE_S3.pdf; FW_Paper_PLOSONE_S4.pdf CALIFORNIA WATER | GROUNDWATER To: GSAs We write to provide a starting point for addressing environmental beneficial users of surface water, as required under the Sustainable Groundwater Management Act (SGMA). SGMA seeks to achieve sustainability, which is defined as the absence of several undesirable results, including “depletions of interconnected surface water that have significant and unreasonable adverse impacts on beneficial users of surface water” (Water Code §10721). The Nature Conservancy (TNC) is a science-based, nonprofit organization with a mission to conserve the lands and waters on which all life depends. Like humans, plants and animals often rely on groundwater for survival, which is why TNC helped develop, and is now helping to implement, SGMA. Earlier this year, we launched the Groundwater Resource Hub, which is an online resource intended to help make it easier and cheaper to address environmental requirements under SGMA. As a first step in addressing when depletions might have an adverse impact, The Nature Conservancy recommends identifying the beneficial users of surface water, which include environmental users. This is a critical step, as it is impossible to define “significant and unreasonable adverse impacts” without knowing what is being impacted. To make this easy, we are providing this letter and the accompanying documents as the best available science on the freshwater species within the boundary of your groundwater sustainability agency (GSA). -

Distribution of Mayfly Species in North America List Compiled from Randolph, Robert Patrick
Page 1 of 19 Distribution of mayfly species in North America List compiled from Randolph, Robert Patrick. 2002. Atlas and biogeographic review of the North American mayflies (Ephemeroptera). PhD Dissertation, Department of Entomology, Purdue University. 514 pages and information presented at Xerces Mayfly Festival, Moscow, Idaho June, 9-12 2005 Acanthametropodidae Ameletus ludens Needham Acanthametropus pecatonica (Burks) Canada—ON,NS,PQ. USA—IL,GA,SC,WI. USA—CT,IN,KY,ME,MO,NY,OH,PA,WV. Ameletus majusculus Zloty Analetris eximia Edmunds Canada—AB. Canada—AB ,SA. USA—MT,OR,WA. USA—UT,WY. Ameletus minimus Zloty & Harper USA—OR. Ameletidae Ameletus oregonenesis McDunnough Ameletus amador Mayo Canada—AB ,BC,SA. Canada—AB. USA—ID,MT,OR,UT. USA—CA,OR. Ameletus pritchardi Zloty Ameletus andersoni Mayo Canada—AB,BC. USA—OR,WA. Ameletus quadratus Zloty & Harper Ameletus bellulus Zloty USA—OR. Canada—AB. Ameletus shepherdi Traver USA—MT. Canada—BC. Ameletus browni McDunnough USA—CA,MT,OR. Canada—PQ Ameletus similior McDunnough USA—ME,PA,VT. Canada—AB,BC. Ameletus celer McDunnough USA—CO,ID,MT,OR,UT Canada—AB ,BC. Ameletus sparsatus McDunnough USA—CO,ID,MT,UT Canada—AB,BC,NWT. Ameletus cooki McDunnough USA—AZ,CO,ID,MT,NM,OR Canada—AB,BC. Ameletus subnotatus Eaton USA—CO,ID,MT,OR,WA. Canada—AB,BC,MB,NB,NF,ON,PQ. Ameletus cryptostimulus Carle USA—CO,UT,WY. USA—NC,NY,PA,SC,TN,VA,VT,WV. Ameletus suffusus McDunnough Ameletus dissitus Eaton Canada—AB,BC. USA—CA,OR. USA—ID,OR. Ameletus doddsianus Zloty Ameletus tarteri Burrows USA—AZ,CO,NM,NV,UT. -

The Mayflies (Ephemeroptera) of Tennessee, with a Review of the Possibly Threatened Species Occurring Within the State
CORE Metadata, citation and similar papers at core.ac.uk Provided by ValpoScholar The Great Lakes Entomologist Volume 29 Number 4 - Summer 1996 Number 4 - Summer Article 1 1996 December 1996 The Mayflies (Ephemeroptera) of Tennessee, With a Review of the Possibly Threatened Species Occurring Within the State L. S. Long Aquatic Resources Center B. C. Kondratieff Colorado State University Follow this and additional works at: https://scholar.valpo.edu/tgle Part of the Entomology Commons Recommended Citation Long, L. S. and Kondratieff, B. C. 1996. "The Mayflies (Ephemeroptera) of Tennessee, With a Review of the Possibly Threatened Species Occurring Within the State," The Great Lakes Entomologist, vol 29 (4) Available at: https://scholar.valpo.edu/tgle/vol29/iss4/1 This Peer-Review Article is brought to you for free and open access by the Department of Biology at ValpoScholar. It has been accepted for inclusion in The Great Lakes Entomologist by an authorized administrator of ValpoScholar. For more information, please contact a ValpoScholar staff member at [email protected]. Long and Kondratieff: The Mayflies (Ephemeroptera) of Tennessee, With a Review of the P 1996 THE GREAT LAKES ENTOMOLOGIST 171 THE MAYFLIES (EPHEMEROPTERA) OF TENNESSEE, WITH A REVIEW OF THE POSSIBLY THREATENED SPECIES OCCURRING WITHIN THE STATE l. S. Long 1 and B. C. Kondratieff2 ABSTRACT One hundred and forty-three species of mayflies are reported from the state of Tennessee. Sixteen species (Ameletus cryptostimuZus, Choroterpes basalis, Baetis virile, Ephemera blanda, E. simulans, Ephemerella berneri, Heterocloeon curiosum, H. petersi, Labiobaetis ephippiatus, Leptophlebia bradleyi, Macdunnoa brunnea, Paraleptophlebia assimilis, P. debilis, P. -
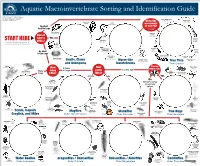
Aquatic Macroinvertebrate Sorting and Identification Guide
Aquatic Macroinvertebrate Sorting and Identification Guide Images courtesy of Troutnut.com, University of Wisconson Extension – ERC Natural Resources Education, University of South Florida College of Education – Florida Center for Instructional Biting Midge Larvae Technology, and Magnus Manske. Ceratopogonidae Midge Larvae Pouch Snails Worm-like Chironomidae Physidae Leeches Order Hirudinea Invertebrate No Shell or True Fly? And No Legs Use images to identify. Zebra Mussels Order Pelecypoda Soldier Fly Larvae Does it Stratiomyidae have a Yes Black Fly Planarians Larvae START HERE Simuliidae Please note that the illustrations are for SHELL? Order Turbellaria Orb reference only and are not true to scale. Snails Order Gastropoda Aquatic Worms Mosquito (Earthworm) Larvae Horse Fly Order Oligochaeta Culicidae Larvae No Shell Tabanidae It Has Legs Aquatic Worms Crane Fly Larvae (Tubifex) Tipulidae Fingernail Clams Order Oligochaeta Order Pelecypoda Horsehair Worms Shore Fly Larvae Snails, Clams Worm-like Order Nematomorpha True Flies Ephydridae and Unknowns Invertebrates Order Diptera Crayfish Piercing Order Decapoda How Water Striders How 3 Pairs MOUTH Part? Gerridae Scuds of Legs 1 or No Tail (Side Swimmer) Many many many Pigmy Order Amphipoda Legs Backswimmers Giant Water Bugs LEGS? TAILS? Pleidae Belostomatidae BEAK 3 Tails 2 Tails Yes Flathead No Mayfly Larvae Identify using Heptageniidae image key Small Squaregill below. Mayfly Larvae Caenidae Giant Stonefly Larvae Pteronarcyidae Isopods (Sowbug) Order Isopoda Spiny Crawler Water Small Water -

Natural Heritage Program List of Rare Animal Species of North Carolina 2020
Natural Heritage Program List of Rare Animal Species of North Carolina 2020 Hickory Nut Gorge Green Salamander (Aneides caryaensis) Photo by Austin Patton 2014 Compiled by Judith Ratcliffe, Zoologist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Animal Species of North Carolina 2020 Compiled by Judith Ratcliffe, Zoologist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. The list is published periodically, generally every two years. -

Appendix 2 Macroinvertebrates 011311
APPENDIX 2 Macroinvertebrates Abstract This appendix reviews the available evidence concerning the potential effects of the activities associated with the Spruce No. 1 Mine on the macroinvertebrate community of receiving streams and presents survey results from streams directly affected by the Spruce No. 1 Mine, including Oldhouse Branch and Pigeonroost Branch, and comparative data from adjacent mined streams impacted by the Dal-Tex operation, including Beech Creek, Left Fork Beech Creek, Rockhouse Creek, and Spruce Fork (Figure A2.1.). Figure A2.1. Map of Spruce No. 1 Mine area and adjacent Dal-Tex operation. EPA conducted three different analyses. First, EPA compared benthic macroinvertebrate community composition from Pigeonroost Branch and Oldhouse Branch to benthic macroinvertebrate community composition from streams that have been impacted by Mingo Logan's Dal-Tex operation. Second, EPA used an observed/expected approach to estimate and quantify taxonomic changes following mining disturbance. Third, EPA compared WVSCI scores in Pigeonroost Branch and Oldhouse Branch with streams impacted by the Dal-Tex operation. The results showed that some naturally occurring taxa will be locally extirpated in the receiving streams and will likely be replaced by pollution-tolerant taxa if mining and filling proceed. These results are supported by the State of West Virginia’s multimetric index (WVSCI), which also indicates that the magnitude and extent of degradation will increase following mining. The appendix also 1 includes a discussion of appropriate invertebrate metrics and data collection and analysis methods and explains why EPA focuses on changes to sensitive taxa and community composition. A2.1. Introduction Macroinvertebrates are good indicators of ecosystem health, and are used by West Virginia and other states in the Mid-Atlantic region and across the U.S. -

Tomah Mayfly Assessment
TOMAH MAYFLY ASSESSMENT February 21, 2001 Dr. K. Elizabeth Gibbs Marcia Siebenmann Dr. Mark McCollough Andrew Weik Beth Swartz MAINE DEPARTMENT OF INLAND FISHERIES AND WILDLIFE WILDLIFE DIVISION RESOURCE ASSESSMENT SECTION ENDANGERED AND THREATENED SPECIES PROGRAM Tomah Mayfly Assessment TABLE OF CONTENTS PAGE INTRODUC TION............................................................................................. 4 NATURAL HISTORY....................................................................................... 5 Description............................................................................................ 5 Distribution............................................................................................ 5 Life History.......................................................................................... 10 MANAGEMENT ............................................................................................ 16 Regulatory Authority ........................................................................... 16 Protection of Maine’s Invertebrates............................................... 16 Protection of Endangered and Threatened Invertebrates ............. 17 Habitat Protection.......................................................................... 19 Section 404 Clean Water Act ........................................... 20 The Maine Endangered Species Act ................................ 21 Natural Resource Protection Act of 1988 ......................... 22 Mandatory Shoreland Zoning .......................................... -

Minesing Wetlands Biological Inventory
Minesing Wetlands Biological Inventory February 2007 Prepared for: Friends of Minesing Wetlands Minesing Wetlands Biological Inventory & Nottawasaga Valley Conservation12/13/2007 Authority Nottawasaga Valley Conservation Authority MINESING WETLANDS BIOLOGICAL INVENTORY Prepared by ROBERT L. BOWLES, JOLENE LAVERTY and DAVID FEATHERSTONE February 2007 Prepared for Friends of Minesing Wetlands & Nottawasaga Valley Conservation Authority Minesing Wetlands Biological Inventory 12/13/2007 Nottawasaga Valley Conservation Authority FOREWARD The Minesing Wetlands Biological Inventory and Evaluation was conducted during 2005-2006 field season. Technical investigations were conducted within the Minesing Wetlands by Bowles Environmental Consultants and Nottawasaga Valley Conservation Authority (NVCA) for the NVCA Minesing Wetlands Management Plan and for Friends of Minesing Wetlands (FOMW). This report received technical review prior to its publication and does not necessarily signify that its contents reflect the views and policies of the Friends of Minesing Wetlands or their partners; nor does mention of trade names or commercial products constitute endorsement or recommendation for use. For additional copies of this report or information about NVCA or FOMW, please contact: Nottawasaga Valley Conservation Authority Centre for Conservation John Hix Conservation Administration Centre 8195 Concession Line 8 Utopia, Ontario L0M 1T0 Phone: (705) 424-1479 Fax: (705) 424-2115 www.nvca.on.ca Friends of Minesing Wetlands www.minesingswamp.ca Minesing Wetlands