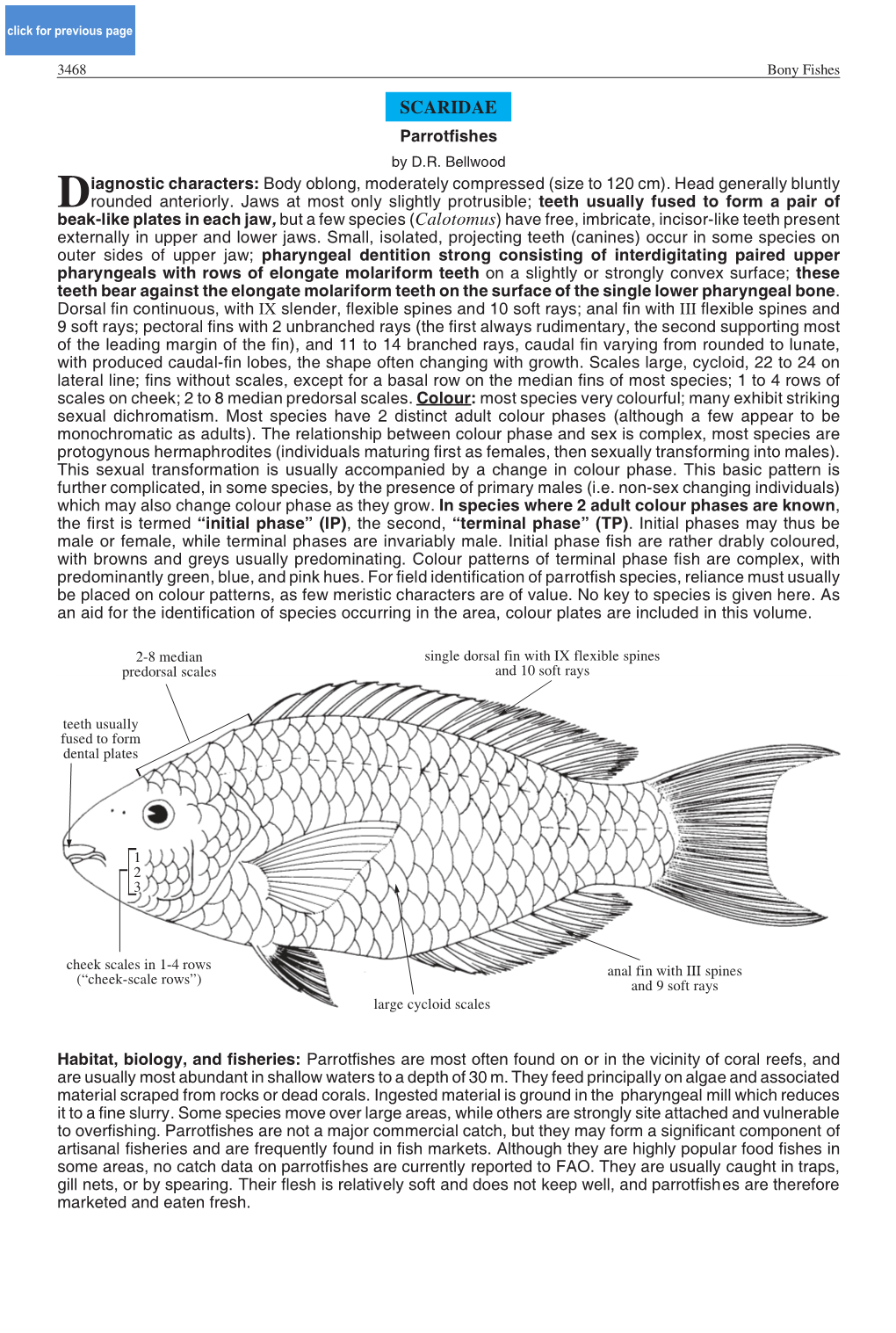
SCARIDAE Parrotfishes by D.R
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Bolbometopon Muricatum) in North Maluku Waters Muhammad J
DNA barcode and phylogenetics of green humphead parrotfish (Bolbometopon muricatum) in North Maluku waters Muhammad J. Achmad, Riyadi Subur, Supyan, Nebuchadnezzar Akbar Faculty of Fisheries and Marine Sciences, Khairun University, Ternate, North Maluku, Indonesia. Corresponding author: N. Akbar, [email protected] Abstract. The green humphead parrotfish (Bolbometopon muricatum) is one of the large species inhabiting coral reefs in North Maluku waters, Indonesia. The declining fish populations due to excessive fishing has caused the green humphead parrotfish to be listed in the Red List of IUCN in the vulnerable category since 2012. The species could be highly endangered, bordering extinction in the future. Studies on the genetic identification of green humphead parrotfish could be considered critical in the policy of sustainable conservation and fish culture. This research is designed for the identification and analysis of the genetic relationship of green humphead parrotfish based on the COI (cytochrome-c-oxidase subunit I) gene. DNA samples were collected from 4 locations in North Maluku, Ternate Island, Morotai Island, Bacan Island and Sanan Island. The DNA from samples was extracted and the COI gene was amplified using PCR (Polymerase Chain Reaction). Furthermore, the amplicon was sequenced to observe the similarities with the NCBI GenBank database. The results of this study showed that the green humphead parrotfish from this study had high similarities (98-100%) with the green humphead parrotfish with the reference access no. KY235362.1. Based on the phylogenetic tree, the green humphead parrotfish originating from North Maluku has a genetic relationship with the green humphead parrotfish from the database, but with different molecular characters. -

Disney•Pixar's “Finding Dory”
Educator’s Guide GRADES 2-6 Created in partnership with the Educational Team isney•Pixar’s “Finding Dory” welcomes back to the big convinced his biological sonar skills are on the fritz; and Dscreen everyone’s favorite forgetful blue tang Dory Destiny (voice of Kaitlin Olson), a nearsighted whale shark. (voice of Ellen DeGeneres), who’s living happily in the reef Deftly navigating the complex inner workings of the MLI, with Marlin (voice of Albert Brooks) and Nemo (voice Dory and her friends discover the magic within their flaws, of Hayden Rolence). When Dory suddenly remembers friendships and family. that she has a family out there who may be looking for Directed by Andrew Stanton (“Finding Nemo,” “WALL•E”), her, the trio takes off on a life-changing adventure across co-directed by Angus MacLane (“Toy Story OF TERROR!”), the ocean to California’s prestigious Marine Life Institute and produced by Lindsey Collins (co-producer “WALL•E”), (MLI), a rehabilitation center and aquarium. In an effort to Disney•Pixar’s “Finding Dory” swims home on Digital find her mom (voice of Diane Keaton) and dad (voice of HD October 25 and on Blu-ray™ November 15. For Eugene Levy), Dory enlists the help of three of the MLI’s more information, like us on Facebook, https://www. most intriguing residents: Hank (voice of Ed O’Neill), a facebook.com/PixarFindingDory, and follow us on Twitter, cantankerous octopus who frequently gives employees https://twitter.com/findingdory and Instagram, https:// the slip; Bailey (voice of Ty Burrell), a beluga whale who is instagram.com/DisneyPixar. -

Life History Compendium of Exploited Hawaiian Fishes
Life History Compendium of Exploited Hawaiian Fishes Prepared for Fisheries Local Action Strategy and Division of Aquatic Resources Prepared by K. Longenecker Hawai‘i Biological Survey Bishop Museum 1525 Bernice Street Honolulu, Hawai‘i 96817 R. Langston Windward Community College 45-720 Keahaala Road Kaneohe, Hawai‘i 96744 July 2008 1 Table of Contents INTRODUCTION .......................................................................................................................... 3 METHODS ..................................................................................................................................... 3 Description of life history parameters: ....................................................................................... 4 RESULTS ....................................................................................................................................... 6 HOLOCENTRIDAE ................................................................................................................... 7 Myripristis amaena (Castelnau, 1873) [3] .............................................................................. 7 Sargocentron diadema (Lacepède, 1802) [13] ..................................................................... 10 CARANGIDAE ........................................................................................................................ 13 Caranx ignobilis (Forsskål, 1775) [17] ................................................................................. 13 Caranx melampygus -

Westminsterresearch Haemogregarina Bigemina
WestminsterResearch http://www.westminster.ac.uk/westminsterresearch Haemogregarina bigemina (Protozoa : Apicomplexa : Adeleorina) – Past, present and future Davies A.J., Smit N.J., Hayes P.M., Seddon A.M. and Wertheim D.F. This is the publisher version of an article published in Folia Parasitologica 51 (2/3) 99- 108 2004. The final definitive version is available online at:https://dx.doi.org/10.14411/fp.2004.015 © 2004 Parazitologický ústav AVČR. The WestminsterResearch online digital archive at the University of Westminster aims to make the research output of the University available to a wider audience. Copyright and Moral Rights remain with the authors and/or copyright owners. Whilst further distribution of specific materials from within this archive is forbidden, you may freely distribute the URL of WestminsterResearch: ((http://westminsterresearch.wmin.ac.uk/). In case of abuse or copyright appearing without permission e-mail [email protected] FOLIA PARASITOLOGICA 51: 99–108, 2004 REVIEW ARTICLE Haemogregarina bigemina (Protozoa: Apicomplexa: Adeleorina) – past, present and future Angela J. Davies1, Nico J. Smit2, Polly M. Hayes1, Alan M. Seddon1 and David Wertheim3 1School of Life Sciences and 3School of Computing, Kingston University, Penrhyn Road, Kingston upon Thames, Surrey, KT1 2EE, UK; 2Department of Zoology, Rand Afrikaans University, P.O. Box 524, Auckland Park 2006, South Africa Key words: Haemogregarina bigemina, haemogregarines, development, transmission, Gnathia, isopods Abstract. This paper reviews past, current and likely future research on the fish haemogregarine, Haemogregarina bigemina Laveran et Mesnil, 1901. Recorded from 96 species of fishes, across 70 genera and 34 families, this broad distribution for H. bigemina is questioned. -

Reef Fishes of the Bird's Head Peninsula, West
Check List 5(3): 587–628, 2009. ISSN: 1809-127X LISTS OF SPECIES Reef fishes of the Bird’s Head Peninsula, West Papua, Indonesia Gerald R. Allen 1 Mark V. Erdmann 2 1 Department of Aquatic Zoology, Western Australian Museum. Locked Bag 49, Welshpool DC, Perth, Western Australia 6986. E-mail: [email protected] 2 Conservation International Indonesia Marine Program. Jl. Dr. Muwardi No. 17, Renon, Denpasar 80235 Indonesia. Abstract A checklist of shallow (to 60 m depth) reef fishes is provided for the Bird’s Head Peninsula region of West Papua, Indonesia. The area, which occupies the extreme western end of New Guinea, contains the world’s most diverse assemblage of coral reef fishes. The current checklist, which includes both historical records and recent survey results, includes 1,511 species in 451 genera and 111 families. Respective species totals for the three main coral reef areas – Raja Ampat Islands, Fakfak-Kaimana coast, and Cenderawasih Bay – are 1320, 995, and 877. In addition to its extraordinary species diversity, the region exhibits a remarkable level of endemism considering its relatively small area. A total of 26 species in 14 families are currently considered to be confined to the region. Introduction and finally a complex geologic past highlighted The region consisting of eastern Indonesia, East by shifting island arcs, oceanic plate collisions, Timor, Sabah, Philippines, Papua New Guinea, and widely fluctuating sea levels (Polhemus and the Solomon Islands is the global centre of 2007). reef fish diversity (Allen 2008). Approximately 2,460 species or 60 percent of the entire reef fish The Bird’s Head Peninsula and surrounding fauna of the Indo-West Pacific inhabits this waters has attracted the attention of naturalists and region, which is commonly referred to as the scientists ever since it was first visited by Coral Triangle (CT). -

Length-Weight Relationships of Thirteen Species of Parrotfish (Family Scaridae) Inhabiting the Egyptian Coasts of the Red Sea
Egyptian Journal of Aquatic Biology & Fisheries Zoology Department, Faculty of Science, Ain Shams University, Cairo, Egypt. ISSN 1110 – 6131 Vol. 23(5): 357 - 366 (2019) www.ejabf.journals.ekb.eg Length-Weight Relationships of Thirteen Species of Parrotfish (Family Scaridae) inhabiting the Egyptian coasts of the Red Sea. Amal M. Amin*, Azza A. El-Ganainy and Manal M. Sabrah National Institute of Oceanography and Fisheries, Suez, Egypt. *Corresponding Author: [email protected] ARTICLE INFO ABSTRACT Article History: Length-weight data of population are basic parameters for any Received: Sept. 8, 2019 monitoring study of fishes since it provides important information about the Accepted: Nov. 27, 2019 structure of the populations. Also, it is important for fish stock assessment Online: Dec. 2019 essential for estimating growth rates, age structure, calculate the standing _______________ stocks biomass, condition indices and several other aspects of fish population dynamics. Therefore, we investigated the length-weight relationships of 13 Keywords: parrotfish species (Family Scaridae) collected seasonally from the Egyptian Red Sea Red Sea coast during 2014/2016. The" b "values of the length-weight Scaridae relationships ranged from 2.17 to 3.88 with a mean value of 2.729±0.0788 Chlorurus geuozonatus (S.E.) for the studied species. Chlorurus geuozonatus showed a positive Calotomus viridescens allometric growth while Calotomus viridescens; Cetoscarus bicolor; Parrotfish Chlorurus sordidus; Chlorurus gibbus; Hipposcarus harid; Scarus frenatus; growth type Scarus ferrugineus; Scarus fuscopurpuerus; Scarus ghobban; scarus niger and Scarus psittacus were show a negative allometric growth. Isometric growth was represented by two species Hipposcarus harid and Scarus colon. 98% of the studied species had "R²" values higher than 0.90, which indicated the increase in length will contribute with increase in weight. -

Effects of Coral Bleaching on Coral Reef Fish Assemblages
Effects of Coral Bleaching on Coral Reef Fish Assemblages Nicholas A J Graham A Thesis submitted to Newcastle University for the Degree of Doctor of Philosophy School of Marine Science and Technology Supervisors: Professor Nicholas V C Polunin Professor John C Bythell Examiners: Professor Matthew G Bentley Dr Magnus Nyström First submitted: 1st July 2008 Viva-Voce: 1st September 2008 Abstract Coral reefs have emerged as one of the ecosystems most vulnerable to climate variation and change. While the contribution of climate warming to the loss of live coral cover has been well documented, the associated effects on fish have not. Such information is important as coral reef fish assemblages provide critical contributions to ecosystem function and services. This thesis assesses the medium to long term impacts of coral loss on fish assemblages in the western Indian Ocean. Feeding observations of corallivorous butterflyfish demonstrates that considerable feeding plasticity occurs among habitat types, but strong relationships exist between degree of specialisation and declines in abundance following coral loss. Furthermore, obligate corallivores are lost fairly rapidly following decline in coral cover, whereas facultative corallivores are sustained until the structure of the dead coral begins to erode. Surveys of benthic and fish assemblages in Mauritius spanning 11 years highlight small changes in both benthos and fish through time, but strong spatial trends associated with dredging and inter-specific competition. In Seychelles, although there was little change in biomass of fishery target species above size of first capture, size spectra analysis of the entire assemblage revealed a loss of smaller individuals (<30cm) and an increase in the larger individuals (>45cm). -

Bhead Parrotfish Listing Petition
PETITION TO LIST THE Bumphead Parrotfish (Bolbometopon muricatum) UNDER THE U.S. ENDANGERED SPECIES ACT Photo: J.E. Maragos, USFWS Petition Submitted to the U.S. Secretary of Commerce, Acting Through the National Oceanic and Atmospheric Administration Fisheries Service & the U.S. Secretary of Interior, Acting through the U.S. Fish and Wildlife Service Petitioner: WildEarth Guardians 312 Montezuma Ave. Santa Fe, New Mexico 87501 (505) 988-9126 December 31, 2009 WildEarth Guardians Petition to List 1 the Bumphead Parrotfish Under the ESA Executive Summary The Bumphead Parrotfish (Bolbometopon muricatum) is a marine fish that feeds primarily on coral. It occurs in many countries in the Pacific and Indo-Pacific, including islands governed by the United States. While wide-ranging, scientists describe it as declining across its range and nearly eliminated from many areas. The primary threat has been overfishing, to which this fish is especially vulnerable due to its behavior of sleeping in large groups at night near reefs. Growing threats are coral bleaching and ocean acidification, both due to climate change. The Bumphead Parrotfish’s fate is tied to coral, as each fish consumes over 5 tons of coral every year. Coral consumed by the Parrotfish is excreted as coral sand, which is important to sustain the coral ecosystem, as well as providing beautiful white sand beaches enjoyed by tourists. Given the economic importance of tourism in the range of the Parrotfish, this species provides an invaluable ecosystem service to humans. An even more important way in which Parrotfish benefit humans is by protecting coral reef ecosystems, which are vital to safeguarding human coastal populations from impacts of extreme weather events. -

Monitoring Functional Groups of Herbivorous Reef Fishes As Indicators of Coral Reef Resilience a Practical Guide for Coral Reef Managers in the Asia Pacifi C Region
Monitoring Functional Groups of Herbivorous Reef Fishes as Indicators of Coral Reef Resilience A practical guide for coral reef managers in the Asia Pacifi c Region Alison L. Green and David R. Bellwood IUCN RESILIENCE SCIENCE GROUP WORKING PAPER SERIES - NO 7 IUCN Global Marine Programme Founded in 1958, IUCN (the International Union for the Conservation of Nature) brings together states, government agencies and a diverse range of non-governmental organizations in a unique world partnership: over 100 members in all, spread across some 140 countries. As a Union, IUCN seeks to influence, encourage and assist societies throughout the world to conserve the integrity and diversity of nature and to ensure that any use of natural resources is equitable and ecologically sustainable. The IUCN Global Marine Programme provides vital linkages for the Union and its members to all the IUCN activities that deal with marine issues, including projects and initiatives of the Regional offices and the six IUCN Commissions. The IUCN Global Marine Programme works on issues such as integrated coastal and marine management, fisheries, marine protected areas, large marine ecosystems, coral reefs, marine invasives and protection of high and deep seas. The Nature Conservancy The mission of The Nature Conservancy is to preserve the plants, animals and natural communities that represent the diversity of life on Earth by protecting the lands and waters they need to survive. The Conservancy launched the Global Marine Initiative in 2002 to protect and restore the most resilient examples of ocean and coastal ecosystems in ways that benefit marine life, local communities and economies. -

Patterns and Processes in the Evolutionary History of Parrotfishes
bs_bs_banner Biological Journal of the Linnean Society, 2012, ••, ••–••. With 5 figures Patterns and processes in the evolutionary history of parrotfishes (Family Labridae) JOHN. H. CHOAT1*, OYA. S. KLANTEN1†, LYNNE VAN HERWERDEN1, D. ROSS ROBERTSON2‡ and KENDALL D. CLEMENTS3 1School of Tropical and Marine Biology, James Cook University, Townsville, QLD, 4811, Australia 2Smithsonian Tropical Research Institute, Ancon, Balboa, Republic of Panama 3School of Biological Sciences, University of Auckland, Private Bag 92019, Auckland 1142, New Zealand Received 5 March 2012; revised 23 May 2012; accepted for publication 23 May 2012 Phylogenetic reconstruction of the evolutionary relationships among 61 of the 70 species of the parrotfish genera Chlorurus and Scarus (Family Labridae) based on mitochondrial and nuclear gene sequences retrieved 15 well-supported clades with mid Pliocene/Pleistocene diversification. Twenty-two reciprocally monophyletic sister- species pairs were identified: 64% were allopatric, and the remainder were sympatric. Age of divergence was similar for allopatric and sympatric species pairs. Sympatric sister pairs displayed greater divergence in morphol- ogy, ecology, and sexually dimorphic colour patterns than did allopatric pairs, suggesting that both genetic drift in allopatric species pairs and ecologically adaptive divergence between members of sympatric pairs have played a role in diversification. Basal species typically have small geographical ranges and are restricted to geographically and ecologically peripheral reef habitats. We found little evidence that a single dominant process has driven diversification, nor did we detect a pattern of discrete, sequential stages of diversification in relation to habitat, ecology, and reproductive biology. The evolution of Chlorurus and Scarus has been complex, involving a number of speciation processes. -

Life on the Coral Reef
Coral Reef Teacher’s Guide Life on the Coral Reef Life on the Coral Reef THE CORAL REEF ECOSYSTEM The muddy silt drifts out to sea, covering the nearby Coral reefs provide the basis for the most productive coral reefs. Some corals can remove the silt, but many shallow water ecosystem in the world. An ecosystem cannot. If the silt is not washed off within a short pe- is a group of living things, such as coral, algae and riod of time by the current, the polyps suffocate and fishes, along with their non-living environment, such die. Not only the rainforest is destroyed, but also the as rocks, water, and sand. Each influences the other, neighboring coral reef. and both are necessary for the successful maintenance of life. If one is thrown out of balance by either natural Reef Zones or human-made causes, then the survival of the other Coral reefs are not uniform, but are shaped by the is seriously threatened. forces of the sea and the structure of the sea floor into DID YOU KNOW? All of the Earth’s ecosystems are a series of different parts or reef zones. Understand- interrelated, forming a shell of life that covers the ing these zones is useful in understanding the ecol- entire planet – the biosphere. For instance, if too many ogy of coral reefs. Keep in mind that these zones can trees are cut down in the rainforest, soil from the for- blend gradually into one another, and that sometimes est is washed by rain into rivers that run to the ocean. -

Annotated Checklist of the Fishes of Wake Atoll1
Annotated Checklist ofthe Fishes ofWake Atoll 1 Phillip S. Lobel2 and Lisa Kerr Lobel 3 Abstract: This study documents a total of 321 fishes in 64 families occurring at Wake Atoll, a coral atoll located at 19 0 17' N, 1660 36' E. Ten fishes are listed by genus only and one by family; some of these represent undescribed species. The first published account of the fishes of Wake by Fowler and Ball in 192 5 listed 107 species in 31 families. This paper updates 54 synonyms and corrects 20 misidentifications listed in the earlier account. The most recent published account by Myers in 1999 listed 122 fishes in 33 families. Our field surveys add 143 additional species records and 22 new family records for the atoll. Zoogeo graphic analysis indicates that the greatest species overlap of Wake Atoll fishes occurs with the Mariana Islands. Several fish species common at Wake Atoll are on the IUCN Red List or are otherwise of concern for conservation. Fish pop ulations at Wake Atoll are protected by virtue of it being a U.S. military base and off limits to commercial fishing. WAKE ATOLL IS an isolated atoll in the cen and Strategic Defense Command. Conse tral Pacific (19 0 17' N, 1660 36' E): It is ap quentially, access has been limited due to the proximately 3 km wide by 6.5 km long and military mission, and as a result the aquatic consists of three islands with a land area of fauna of the atoll has not received thorough 2 approximately 6.5 km • Wake is separated investigation.