NIH Public Access Author Manuscript Lasers Surg Med
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Dermatology Eponyms – Sign –Lexicon (P)
2XU'HUPDWRORJ\2QOLQH Historical Article Dermatology Eponyms – sign –Lexicon (P)� Part 2 Piotr Brzezin´ ski1,2, Masaru Tanaka3, Husein Husein-ElAhmed4, Marco Castori5, Fatou Barro/Traoré6, Satish Kashiram Punshi7, Anca Chiriac8,9 1Department of Dermatology, 6th Military Support Unit, Ustka, Poland, 2Institute of Biology and Environmental Protection, Department of Cosmetology, Pomeranian Academy, Slupsk, Poland, 3Department of Dermatology, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan, 4Department of Dermatology, San Cecilio University Hospital, Granada, Spain, 5Medical Genetics, Department of Experimental Medicine, Sapienza - University of Rome, San Camillo-Forlanini Hospital, Rome, Italy, 6Department of Dermatology-Venerology, Yalgado Ouédraogo Teaching Hospital Center (CHU-YO), Ouagadougou, Burkina Faso, 7Consultant in Skin Dieseases, VD, Leprosy & Leucoderma, Rajkamal Chowk, Amravati – 444 601, India, 8Department of Dermatology, Nicolina Medical Center, Iasi, Romania, 9Department of Dermato-Physiology, Apollonia University Iasi, Strada Muzicii nr 2, Iasi-700399, Romania Corresponding author: Piotr Brzezin′ski, MD PhD, E-mail: [email protected] ABSTRACT Eponyms are used almost daily in the clinical practice of dermatology. And yet, information about the person behind the eponyms is difficult to find. Indeed, who is? What is this person’s nationality? Is this person alive or dead? How can one find the paper in which this person first described the disease? Eponyms are used to describe not only disease, but also clinical signs, surgical procedures, staining techniques, pharmacological formulations, and even pieces of equipment. In this article we present the symptoms starting with (P) and other. The symptoms and their synonyms, and those who have described this symptom or phenomenon. Key words: Eponyms; Skin diseases; Sign; Phenomenon Port-Light Nose sign or tylosis palmoplantaris is widely related with the onset of squamous cell carcinoma of the esophagus. -

Guide to Policy & Practice Questions
OREGON BOARD OF CHIROPRACTIC EXAMINERS GUIDE TO POLICY & PRACTICE QUESTIONS 530 Center St NE, suite 620 Salem, OR 97301 (503) 378-5816 [email protected] Updated/Adopted: 9/17/2020 TABLE OF CONTENTS SECTION I ............................................................................................................................................................................................... 6 DEVICES, PROCEDURES, AND SUBSTANCES ............................................................................................................................... 6 DEVICES ................................................................................................................................................................ 6 BAX 3000 AND SIMILAR DEVICES................................................................................................................................................ 6 BIOPTRON LIGHT THERAPY ........................................................................................................................................................ 6 CPAP MACHINE, ORDERING ....................................................................................................................................................... 6 CTD MARK I MULTI-TORSION TRACTION DEVICE................................................................................................................... 6 DYNATRON 2000 ........................................................................................................................................................................... -

Clinicopathological Correlation of Acquired Hyperpigmentary Disorders
Symposium Clinicopathological correlation of acquired Dermatopathology hyperpigmentary disorders Anisha B. Patel, Raj Kubba1, Asha Kubba1 Department of Dermatology, ABSTRACT Oregon Health Sciences University, Portland, Oregon, Acquired pigmentary disorders are group of heterogenous entities that share single, most USA, 1Delhi Dermatology Group, Delhi Dermpath significant, clinical feature, that is, dyspigmentation. Asians and Indians, in particular, are mostly Laboratory, New Delhi, India affected. Although the classic morphologies and common treatment options of these conditions have been reviewed in the global dermatology literature, the value of histpathological evaluation Address for correspondence: has not been thoroughly explored. The importance of accurate diagnosis is emphasized here as Dr. Asha Kubba, the underlying diseases have varying etiologies that need to be addressed in order to effectively 10, Aradhana Enclave, treat the dyspigmentation. In this review, we describe and discuss the utility of histology in the R.K. Puram, Sector‑13, diagnostic work of hyperpigmentary disorders, and how, in many cases, it can lead to targeted New Delhi ‑ 110 066, India. E‑mail: and more effective therapy. We focus on the most common acquired pigmentary disorders [email protected] seen in Indian patients as well as a few uncommon diseases with distinctive histological traits. Facial melanoses, including mimickers of melasma, are thoroughly explored. These diseases include lichen planus pigmentosus, discoid lupus erythematosus, drug‑induced melanoses, hyperpigmentation due to exogenous substances, acanthosis nigricans, and macular amyloidosis. Key words: Facial melanoses, histology of hyperpigmentary disorders and melasma, pigmentary disorders INTRODUCTION focus on the most common acquired hyperpigmentary disorders seen in Indian patients as well as a few Acquired pigmentary disorders are found all over the uncommon diseases with distinctive histological traits. -
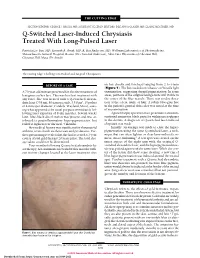
Q-Switched Laser-Induced Chrysiasis Treated with Long-Pulsed Laser
THE CUTTING EDGE SECTION EDITOR: GEORGE J. HRUZA, MD; ASSISTANT SECTION EDITORS: DEE ANNA GLASER, MD; ELAINE SIEGFRIED, MD Q-Switched Laser-Induced Chrysiasis Treated With Long-Pulsed Laser Patricia Lee Yun, MD; Kenneth A. Arndt, MD; R. Rox Anderson, MD; Wellman Laboratories of Photomedicine, Massachusetts General Hospital, Boston (Drs Yun and Anderson), Skin Care Physicians of Chestnut Hill, Chestnut Hill, Mass (Dr Arndt) The Cutting Edge: Challenges in Medical and Surgical Therapeutics REPORT OF A CASE on her cheeks and forehead ranging from 2 to 6 mm (Figure 1). The lesions did not enhance on Wood’s light A 70-year-old woman presented for elective treatment of examination, suggesting dermal pigmentation. In some lentigines on her face. This was her first treatment with areas, portions of the original lentigo were still visible in any laser. She was treated with a Q-switched alexan- the center of the blue macule. There was no discolora- drite laser (755 nm, 50 nanoseconds, 3.5 J/cm2, 15 pulses tion of the sclera, nails, or hair. A subtle blue-gray hue of 4-mm spot diameter; Candela, Wayland, Mass), caus- in the patient’s general skin color was noted at the time ing what appeared to be usual purpura immediately fol- of reexamination. lowing laser exposure of 8 tan macules. Several weeks A punch biopsy specimen of a representative area dem- later, blue-black discoloration was present, and was at- onstrated numerous black particles within macrophages tributed to postinflammatory hyperpigmentation, but in the dermis. A diagnosis of Q-switched laser-induced failed to lighten over the next 4 months. -

Hydroxychloroquine-Associated Hyperpigmentation Mimicking Elder Abuse
Dermatol Ther (Heidelb) (2013) 3:203–210 DOI 10.1007/s13555-013-0032-z CASE REPORT Hydroxychloroquine-Associated Hyperpigmentation Mimicking Elder Abuse Philip R. Cohen To view enhanced content go to www.dermtherapy-open.com Received: June 17, 2013 / Published online: August 14, 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com ABSTRACT cleared of suspected elder abuse. A skin biopsy of the patient’s dyschromia confirmed the Background: Hydroxychloroquine may result diagnosis of hydroxychloroquine-associated in cutaneous dyschromia. Older individuals hyperpigmentation. who are the victims of elder abuse can present Conclusion: Hyperpigmentation of skin, with bruising and resolving ecchymoses. mucosa, and nails can be observed in patients Purpose: The features of hydroxychloroquine- treated with antimalarials, including associated hyperpigmentation are described, hydroxychloroquine. Elder abuse is a significant the mucosal and skin manifestations of elder and underreported problem in seniors. abuse are reviewed, and the mucocutaneous Cutaneous findings can aid in the discovery of mimickers of elder abuse are summarized. physical abuse, sexual abuse, and self-neglect in Case Report: An elderly woman being treated elderly individuals. However, medication- with hydroxychloroquine for systemic lupus associated effects, systemic conditions, and erythematosus developed drug-associated black accidental external injuries can mimic elder and blue pigmentation of her skin. The abuse. Therefore, a complete medical history dyschromia was misinterpreted by her and appropriate laboratory evaluation, including clinician as elder abuse and Adult Protective skin biopsy, should be conducted when the Services was notified. The family was eventually diagnosis of elder abuse is suspected. Keywords: Abuse; Dyschromia; Elderly; P. -

Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal
ytology & f C H o is Prakash, et al., J Cytol Histol 2018, 9:2 l t a o n l o r DOI: 10.4172/2157-7099.1000501 g u y o J Journal of Cytology & Histology ISSN: 2157-7099 Research Article Open Access Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal Silver in Primate Vertebrates Pani Jyoti Prakash*1, Singh Royana2 and Pani Sankarsan3 1Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India 2Department of Anatomy, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttarpradesh, India 3Deapartment of Surgery, Institute of Medical Science and Research, Karjat, Bhivpuri, India *Corresponding author: Prakash PJ, Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India, Tel: 8433668356; E-mail: [email protected] Received date: February 21, 2018; Accepted date: March 12, 2018; Published date: March 16, 2018 Copyright: © 2018 Prakash PJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Back ground: In this most recent, update global arena for consumers products most of the daily applications of bigger silver nano particles (20 to 100 nano meter range) are effected as anti-viral and anti-parasitic agents in clinical medicine and diagnosis which is a positive feedback. However, the major negative feedback of bigger size silver nano particles on human, animal and primate vertebrate body is multisystem teratogenicity focuses. Material and methods: This study was designed to investigate teratogenic effects of bigger size nano silver which is poly vinyl pyrollidone coated and sodium borohydride stabilized. -
Ocular Chrysiasis: a Teaching Case Report | 1
Ocular Chrysiasis: a Teaching Case Report | 1 Abstract Ocular chrysiasis is a deposition of gold in ocular structures secondary to gold salt therapy, which is primarily used in infectious, rheumatoid and psoriatic arthritis. Gold therapy has become an extremely rare treatment due to the advent of rheumatologic medications with better safety profiles. However, any patient who has undergone gold therapy could potentially have ocular chrysiasis. Therefore, it must be included as a differential in the presence of corneal or lenticular changes in these patients. Additionally, it is important to include ocular chrysiasis as a differential in the presence of crystalline keratopathies. A detailed medical history and thorough ocular examination can assist clinicians in making a correct diagnosis. Key Words: gold salts, ocular chrysiasis, autoimmune disease, cornea Background The following case report is meant to be used as a guide in teaching optometry students and residents. It is relevant to all levels of training. Ocular chrysiasis is a deposition of gold in ocular structures following chrysotherapy, which is the medical use of gold salts. Chrysotherapy was primarily used to treat infectious, rheumatoid and psoriatic arthritis. This therapy has become an extremely rare treatment due to the advent of rheumatologic medications with better safety profiles. Any patient who has undergone gold therapy could potentially have ocular chrysiasis. Therefore, it must be included as a differential in the presence of corneal or lenticular changes in these patients. This case illustrates the importance of history-taking skills, examination and decision-making in effectively diagnosing this condition. Case Description A 54-year-old Caucasian female presented for an annual eye examination. -

A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient with Comorbid Schizoaffective Disorder
A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient With Comorbid Schizoaffective Disorder Sarah J. Schrauben, MD; Dhaval G. Bhanusali, MD; Stuart Sheets, MD; Animesh A. Sinha, MD, PhD Argyria is a rare cutaneous manifestation of silver is an elemental compound often found in drinking deposits in the skin, characterized by a grayish water, various sources in industry, and alternative blue discoloration, particularly in sun-exposed medicine compounds. Silver was first used medically areas. We report the case of a patient with a his- in 980 ad; it was believed to provide a means of tory of schizoaffective disorder and type 2 diabe- blood purification, to treat heart palpitations, and to tes mellitus who presented with argyria of the face serve as an adjunct treatment of epilepsy and tabes and neck. The patient had a history of ingesting dorsalis.1 In the 1900s, silver began being used as a colloidal silver proteins (CSPs) for approximately popular treatment of infections. However, reports of 10 years as a self-prescribed remedy for his medi- unsolicited side effects diminished its popularity as a cal conditions. CUTISmainstay solution for many ailments. Silver recently Colloidal silver protein has gained popularity has regained popularity, with an increase in Internet among patients who seek alternative medical ther- claims promoting the use of oral colloidal silver pro- apies. Argyria is the most predominant manifesta- teins (CSPs) as mineral supplements and as a way to tion of silver toxicity. It is unclear if our patient prevent and treat many diseases.2 began taking CSP becauseDo of his schizoaffectiveNot WeCopy present a patient with argyria as a conse- disorder or if silver toxicity may have induced quence of multiple forms of silver ingestion in an somatic delusions; however, it is important for attempt to treat his type 2 diabetes mellitus and physicians to have a thorough understanding of schizoaffective disorder. -
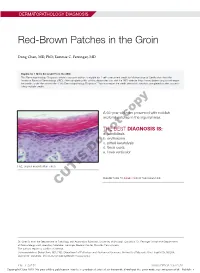
Red-Brown Patches in the Groin
DERMATOPATHOLOGY DIAGNOSIS Red-Brown Patches in the Groin Dong Chen, MD, PhD; Tammie C. Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 66-year-old man presented with reddish arciform patchescopy in the inguinal area. THE BEST DIAGNOSIS IS: a. candidiasis b. noterythrasma c. pitted keratolysis d. tinea cruris Doe. tinea versicolor H&E, original magnification ×600. PLEASE TURN TO PAGE 419 FOR THE DIAGNOSIS CUTIS Dr. Chen is from the Department of Pathology and Anatomical Sciences, University of Missouri, Columbia. Dr. Ferringer is from the Departments of Dermatology and Laboratory Medicine, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Dong Chen, MD, PhD, Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Dr, MA204, DC018.00, Columbia, MO 65212 ([email protected]). 416 I CUTIS® WWW.MDEDGE.COM/CUTIS Copyright Cutis 2018. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Erythrasma rythrasma usually involves intertriginous areas surface (Figure 1) compared to dermatophyte hyphae that (eg, axillae, groin, inframammary area). Patients tend to be parallel to the surface.2 E present with well-demarcated, minimally scaly, red- Pitted keratolysis is a superficial bacterial infection brown patches. -

Tattooed Skin and Health
Current Problems in Dermatology Editors: P. Itin, G.B.E. Jemec Vol. 48 Tattooed Skin and Health Editors J. Serup N. Kluger W. Bäumler Tattooed Skin and Health Current Problems in Dermatology Vol. 48 Series Editors Peter Itin Basel Gregor B.E. Jemec Roskilde Tattooed Skin and Health Volume Editors Jørgen Serup Copenhagen Nicolas Kluger Helsinki Wolfgang Bäumler Regensburg 110 figures, 85 in color, and 25 tables, 2015 Basel · Freiburg · Paris · London · New York · Chennai · New Delhi · Bangkok · Beijing · Shanghai · Tokyo · Kuala Lumpur · Singapore · Sydney Current Problems in Dermatology Prof. Jørgen Serup Dr. Nicolas Kluger Bispebjerg University Hospital Department of Skin and Allergic Diseases Department of Dermatology D Helsinki University Central Hospital Copenhagen (Denmark) Helsinki (Finland) Prof. Wolfgang Bäumler Department of Dermatology University of Regensburg Regensburg (Germany) Library of Congress Cataloging-in-Publication Data Tattooed skin and health / volume editors, Jørgen Serup, Nicolas Kluger, Wolfgang Bäumler. p. ; cm. -- (Current problems in dermatology, ISSN 1421-5721 ; vol. 48) Includes bibliographical references and indexes. ISBN 978-3-318-02776-1 (hard cover : alk. paper) -- ISBN 978-3-318-02777-8 (electronic version) I. Serup, Jørgen, editor. II. Kluger, Nicolas, editor. III. Bäumler, Wolfgang, 1959- , editor. IV. Series: Current problems in dermatology ; v. 48. 1421-5721 [DNLM: 1. Tattooing--adverse effects. 2. Coloring Agents. 3. Epidermis--pathology. 4. Tattooing--legislation & jurisprudence. 5. Tattooing--methods. W1 CU804L v.48 2015 / WR 140] GT2345 391.6’5--dc23 2015000919 Bibliographic Indices. This publication is listed in bibliographic services, including MEDLINE/Pubmed. Disclaimer. The statements, opinions and data contained in this publication are solely those of the individual authors and contributors and not of the publisher and the editor(s). -

Polarized Light Therapy” Cikkek Kivonatai
PubMed „polarized light therapy” cikkek kivonatai 1. Masui. 2009 Nov;58(11):1401-6. [Phototherapy for chronic pain treatment] [Article in Japanese] Ide Y. Department of Anesthesia, Toho University Sakura Medical Center Sakura 285-8741. Three types of machines are used in the field of phototherapy for chronic pain. One type is an instrument for low reactive level laser therapy (LLLT), one is an instrument for linear polarized infrared light irradiation (SUPER LIZER), and the last one is an instrument for Xenon light irradiation (beta EXCEL Xe10). The available machines for LLLT all project laser by semiconductor. The newest machine (MEDILASER SOFT PULSE10) has peak power of 10 W and mean power of 1 W. This machine is as safe as 1 W machine and is effective twice as deep as the 1 W machine. The irradiation by low reactive level laser induces hyperpolarization, decreased resistance of neuronal membrane, and increased intra-cellular ATP concentrations. The effects of low reactive level laser might be induced by the activation of ATP-dependent K channel. The significant analgesic effects of 1 W and 10 W LLLT were reported with double blind test. The significant analgesic effects of linear polarized near infrared light irradiation with double blind test were also reported. The effects of low reactive level laser upon the sympathetic nerve system were thought to result from its normalization of the overloaded sympathetic nerve system. PMID: 19928507 [PubMed - indexed for MEDLINE] 2. Photomed Laser Surg. 2009 Oct 26. [Epub ahead of print] Healing of Surgical Wounds Made with lambda970-nm Diode Laser Associated or Not with Laser Phototherapy (lambda655 nm) or Polarized Light (lambda400-2000 nm). -

Health Hazard Evaluation Report 1981-0036-1023
Health Hazard HETA 81-036-1023 Evaluation ALASKA SMELTING &REFINING COMPANY Report WISILLAJ ALASKA PREFACE The Hazard Evaluations and Technical Assistance Branch of NIOSH conducts field investigations of possible health hazards in the workplace. These investigations are conducted under the authority of Section 20(a)(6) of the Occupational Safety and Health Act of 1970, 29 U.S .C. 669(a)(6} which authorizes the Secretary of Health and Human Services, following a written request from any employer or authorized representative of employees, to detennine whether any substance nonnally found in the place of employment has potentially toxic effects in such concentrations as used or found. The Hazard Evaluations and Technical Assistance Branch also provides, upon request, medical, nursing, and industrial hygiene technical and consultative assistance (TA) to Federal, state, and local agencies; labor; industry and other groups or individuals to control occupational health hazards and to prevent -related trauma and disease. i I l J Mention of company names or products does not constitute endorsement by the It National Institute for Occupational Safety and Health. ~ I f f HETA 81-036-1023 NIOSH INVESTIGATOR: December 1981 . Arvin G. Apol ALASKA SMELTING &REFINING Company Wisilla, Alaska I SUMMARY In November 1980, the National Institute for Occupational Safety and Health (NIOSH) received a request from the Alaska Smelting and Refining Company, Wisilla, Alaska, to determine if a health hazard existed as a result of the three workers' exposures to lead and silver fumes, dust, and chemicals found in the silver smelting and refining process. NIOSH conducted an environmental and medical evaluation on April 28-May 1, 1981.