1, Introduction Introduction
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Melanocytes and Their Diseases
Downloaded from http://perspectivesinmedicine.cshlp.org/ on October 2, 2021 - Published by Cold Spring Harbor Laboratory Press Melanocytes and Their Diseases Yuji Yamaguchi1 and Vincent J. Hearing2 1Medical, AbbVie GK, Mita, Tokyo 108-6302, Japan 2Laboratory of Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892 Correspondence: [email protected] Human melanocytes are distributed not only in the epidermis and in hair follicles but also in mucosa, cochlea (ear), iris (eye), and mesencephalon (brain) among other tissues. Melano- cytes, which are derived from the neural crest, are unique in that they produce eu-/pheo- melanin pigments in unique membrane-bound organelles termed melanosomes, which can be divided into four stages depending on their degree of maturation. Pigmentation production is determined by three distinct elements: enzymes involved in melanin synthesis, proteins required for melanosome structure, and proteins required for their trafficking and distribution. Many genes are involved in regulating pigmentation at various levels, and mutations in many of them cause pigmentary disorders, which can be classified into three types: hyperpigmen- tation (including melasma), hypopigmentation (including oculocutaneous albinism [OCA]), and mixed hyper-/hypopigmentation (including dyschromatosis symmetrica hereditaria). We briefly review vitiligo as a representative of an acquired hypopigmentation disorder. igments that determine human skin colors somes can be divided into four stages depend- Pinclude melanin, hemoglobin (red), hemo- ing on their degree of maturation. Early mela- siderin (brown), carotene (yellow), and bilin nosomes, especially stage I melanosomes, are (yellow). Among those, melanins play key roles similar to lysosomes whereas late melanosomes in determining human skin (and hair) pigmen- contain a structured matrix and highly dense tation. -

Dermatology Eponyms – Sign –Lexicon (P)
2XU'HUPDWRORJ\2QOLQH Historical Article Dermatology Eponyms – sign –Lexicon (P)� Part 2 Piotr Brzezin´ ski1,2, Masaru Tanaka3, Husein Husein-ElAhmed4, Marco Castori5, Fatou Barro/Traoré6, Satish Kashiram Punshi7, Anca Chiriac8,9 1Department of Dermatology, 6th Military Support Unit, Ustka, Poland, 2Institute of Biology and Environmental Protection, Department of Cosmetology, Pomeranian Academy, Slupsk, Poland, 3Department of Dermatology, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan, 4Department of Dermatology, San Cecilio University Hospital, Granada, Spain, 5Medical Genetics, Department of Experimental Medicine, Sapienza - University of Rome, San Camillo-Forlanini Hospital, Rome, Italy, 6Department of Dermatology-Venerology, Yalgado Ouédraogo Teaching Hospital Center (CHU-YO), Ouagadougou, Burkina Faso, 7Consultant in Skin Dieseases, VD, Leprosy & Leucoderma, Rajkamal Chowk, Amravati – 444 601, India, 8Department of Dermatology, Nicolina Medical Center, Iasi, Romania, 9Department of Dermato-Physiology, Apollonia University Iasi, Strada Muzicii nr 2, Iasi-700399, Romania Corresponding author: Piotr Brzezin′ski, MD PhD, E-mail: [email protected] ABSTRACT Eponyms are used almost daily in the clinical practice of dermatology. And yet, information about the person behind the eponyms is difficult to find. Indeed, who is? What is this person’s nationality? Is this person alive or dead? How can one find the paper in which this person first described the disease? Eponyms are used to describe not only disease, but also clinical signs, surgical procedures, staining techniques, pharmacological formulations, and even pieces of equipment. In this article we present the symptoms starting with (P) and other. The symptoms and their synonyms, and those who have described this symptom or phenomenon. Key words: Eponyms; Skin diseases; Sign; Phenomenon Port-Light Nose sign or tylosis palmoplantaris is widely related with the onset of squamous cell carcinoma of the esophagus. -

Guide to Policy & Practice Questions
OREGON BOARD OF CHIROPRACTIC EXAMINERS GUIDE TO POLICY & PRACTICE QUESTIONS 530 Center St NE, suite 620 Salem, OR 97301 (503) 378-5816 [email protected] Updated/Adopted: 9/17/2020 TABLE OF CONTENTS SECTION I ............................................................................................................................................................................................... 6 DEVICES, PROCEDURES, AND SUBSTANCES ............................................................................................................................... 6 DEVICES ................................................................................................................................................................ 6 BAX 3000 AND SIMILAR DEVICES................................................................................................................................................ 6 BIOPTRON LIGHT THERAPY ........................................................................................................................................................ 6 CPAP MACHINE, ORDERING ....................................................................................................................................................... 6 CTD MARK I MULTI-TORSION TRACTION DEVICE................................................................................................................... 6 DYNATRON 2000 ........................................................................................................................................................................... -

Clinicopathological Correlation of Acquired Hyperpigmentary Disorders
Symposium Clinicopathological correlation of acquired Dermatopathology hyperpigmentary disorders Anisha B. Patel, Raj Kubba1, Asha Kubba1 Department of Dermatology, ABSTRACT Oregon Health Sciences University, Portland, Oregon, Acquired pigmentary disorders are group of heterogenous entities that share single, most USA, 1Delhi Dermatology Group, Delhi Dermpath significant, clinical feature, that is, dyspigmentation. Asians and Indians, in particular, are mostly Laboratory, New Delhi, India affected. Although the classic morphologies and common treatment options of these conditions have been reviewed in the global dermatology literature, the value of histpathological evaluation Address for correspondence: has not been thoroughly explored. The importance of accurate diagnosis is emphasized here as Dr. Asha Kubba, the underlying diseases have varying etiologies that need to be addressed in order to effectively 10, Aradhana Enclave, treat the dyspigmentation. In this review, we describe and discuss the utility of histology in the R.K. Puram, Sector‑13, diagnostic work of hyperpigmentary disorders, and how, in many cases, it can lead to targeted New Delhi ‑ 110 066, India. E‑mail: and more effective therapy. We focus on the most common acquired pigmentary disorders [email protected] seen in Indian patients as well as a few uncommon diseases with distinctive histological traits. Facial melanoses, including mimickers of melasma, are thoroughly explored. These diseases include lichen planus pigmentosus, discoid lupus erythematosus, drug‑induced melanoses, hyperpigmentation due to exogenous substances, acanthosis nigricans, and macular amyloidosis. Key words: Facial melanoses, histology of hyperpigmentary disorders and melasma, pigmentary disorders INTRODUCTION focus on the most common acquired hyperpigmentary disorders seen in Indian patients as well as a few Acquired pigmentary disorders are found all over the uncommon diseases with distinctive histological traits. -

Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal
ytology & f C H o is Prakash, et al., J Cytol Histol 2018, 9:2 l t a o n l o r DOI: 10.4172/2157-7099.1000501 g u y o J Journal of Cytology & Histology ISSN: 2157-7099 Research Article Open Access Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal Silver in Primate Vertebrates Pani Jyoti Prakash*1, Singh Royana2 and Pani Sankarsan3 1Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India 2Department of Anatomy, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttarpradesh, India 3Deapartment of Surgery, Institute of Medical Science and Research, Karjat, Bhivpuri, India *Corresponding author: Prakash PJ, Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India, Tel: 8433668356; E-mail: [email protected] Received date: February 21, 2018; Accepted date: March 12, 2018; Published date: March 16, 2018 Copyright: © 2018 Prakash PJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Back ground: In this most recent, update global arena for consumers products most of the daily applications of bigger silver nano particles (20 to 100 nano meter range) are effected as anti-viral and anti-parasitic agents in clinical medicine and diagnosis which is a positive feedback. However, the major negative feedback of bigger size silver nano particles on human, animal and primate vertebrate body is multisystem teratogenicity focuses. Material and methods: This study was designed to investigate teratogenic effects of bigger size nano silver which is poly vinyl pyrollidone coated and sodium borohydride stabilized. -

A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient with Comorbid Schizoaffective Disorder
A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient With Comorbid Schizoaffective Disorder Sarah J. Schrauben, MD; Dhaval G. Bhanusali, MD; Stuart Sheets, MD; Animesh A. Sinha, MD, PhD Argyria is a rare cutaneous manifestation of silver is an elemental compound often found in drinking deposits in the skin, characterized by a grayish water, various sources in industry, and alternative blue discoloration, particularly in sun-exposed medicine compounds. Silver was first used medically areas. We report the case of a patient with a his- in 980 ad; it was believed to provide a means of tory of schizoaffective disorder and type 2 diabe- blood purification, to treat heart palpitations, and to tes mellitus who presented with argyria of the face serve as an adjunct treatment of epilepsy and tabes and neck. The patient had a history of ingesting dorsalis.1 In the 1900s, silver began being used as a colloidal silver proteins (CSPs) for approximately popular treatment of infections. However, reports of 10 years as a self-prescribed remedy for his medi- unsolicited side effects diminished its popularity as a cal conditions. CUTISmainstay solution for many ailments. Silver recently Colloidal silver protein has gained popularity has regained popularity, with an increase in Internet among patients who seek alternative medical ther- claims promoting the use of oral colloidal silver pro- apies. Argyria is the most predominant manifesta- teins (CSPs) as mineral supplements and as a way to tion of silver toxicity. It is unclear if our patient prevent and treat many diseases.2 began taking CSP becauseDo of his schizoaffectiveNot WeCopy present a patient with argyria as a conse- disorder or if silver toxicity may have induced quence of multiple forms of silver ingestion in an somatic delusions; however, it is important for attempt to treat his type 2 diabetes mellitus and physicians to have a thorough understanding of schizoaffective disorder. -
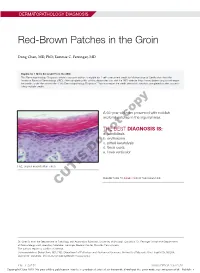
Red-Brown Patches in the Groin
DERMATOPATHOLOGY DIAGNOSIS Red-Brown Patches in the Groin Dong Chen, MD, PhD; Tammie C. Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 66-year-old man presented with reddish arciform patchescopy in the inguinal area. THE BEST DIAGNOSIS IS: a. candidiasis b. noterythrasma c. pitted keratolysis d. tinea cruris Doe. tinea versicolor H&E, original magnification ×600. PLEASE TURN TO PAGE 419 FOR THE DIAGNOSIS CUTIS Dr. Chen is from the Department of Pathology and Anatomical Sciences, University of Missouri, Columbia. Dr. Ferringer is from the Departments of Dermatology and Laboratory Medicine, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Dong Chen, MD, PhD, Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Dr, MA204, DC018.00, Columbia, MO 65212 ([email protected]). 416 I CUTIS® WWW.MDEDGE.COM/CUTIS Copyright Cutis 2018. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Erythrasma rythrasma usually involves intertriginous areas surface (Figure 1) compared to dermatophyte hyphae that (eg, axillae, groin, inframammary area). Patients tend to be parallel to the surface.2 E present with well-demarcated, minimally scaly, red- Pitted keratolysis is a superficial bacterial infection brown patches. -

The University of Chicago Genetic Services Laboratories Labolaboratories
The University of Chicago Genetic Services Laboratories LaboLaboratories5841 S. Maryland Ave., Rm. G701, MC 0077, Chicago, Illinois 60637 3637 [email protected] dnatesting.uchicago.edu CLIA #: 14D0917593 CAP #: 18827-49 Next Generation Sequencing Panel for Albinism Clinical Features: Albinism is a group of inherited disorders in which melanin biosynthesis is reduced or absent [1]. The lack or reduction in pigment can affect the eyes, skin and hair, or only the eyes. In addition, there are several syndromic forms of albinism in which the hypopigmented and visual phenotypes are seen in addition to other systems involvement [2]. Our Albinism Sequencing Panel includes sequence analysis of all 20 genes listed below. Our Albinism Deletion/Duplication Panel includes sequence analysis of all 20 genes listed below. Albinism Sequencing Panel Chediak- Griscelli Oculocutaneous Ocular Hermansky Pudlak syndrome Higashi syndrome Albinism Albinism syndrome TYR SLC45A2 GPR143 HPS1 HPS4 DTNBP1 LYST MYO5A OCA2 SLC24A5 AP3B1 HPS5 BLOC1S3 RAB27A TYRP1 C10ORF11 HPS3 HPS6 BLOC1S6 MLPH Oculocutaneous Albinism Oculocutaneous albinism (OCA) is a genetically heterogeneous congenital disorder characterized by decreased or absent pigmentation in the hair, skin, and eyes. Clinical features can include varying degrees of congenital nystagmus, hypopigmentation and translucency, reduced pigmentation of the retinal pigment epithelium and foveal hypoplasia. Vision acuity is typically reduced and refractive errors, color vision impairment and photophobia also occur [3]. Gene Clinical Features Details TYR Albinism, OCA1 is caused by mutations in the tyrosinase gene, TYR. Mutations completely oculocutaneous, abolishing tyrosinase activity result in OCA1A, while mutations rendering some type I enzyme activity result in OCA1B allowing some accumulation of melanin pigment production throughout life. -

Orphanet Report Series Rare Diseases Collection
Marche des Maladies Rares – Alliance Maladies Rares Orphanet Report Series Rare Diseases collection DecemberOctober 2013 2009 List of rare diseases and synonyms Listed in alphabetical order www.orpha.net 20102206 Rare diseases listed in alphabetical order ORPHA ORPHA ORPHA Disease name Disease name Disease name Number Number Number 289157 1-alpha-hydroxylase deficiency 309127 3-hydroxyacyl-CoA dehydrogenase 228384 5q14.3 microdeletion syndrome deficiency 293948 1p21.3 microdeletion syndrome 314655 5q31.3 microdeletion syndrome 939 3-hydroxyisobutyric aciduria 1606 1p36 deletion syndrome 228415 5q35 microduplication syndrome 2616 3M syndrome 250989 1q21.1 microdeletion syndrome 96125 6p subtelomeric deletion syndrome 2616 3-M syndrome 250994 1q21.1 microduplication syndrome 251046 6p22 microdeletion syndrome 293843 3MC syndrome 250999 1q41q42 microdeletion syndrome 96125 6p25 microdeletion syndrome 6 3-methylcrotonylglycinuria 250999 1q41-q42 microdeletion syndrome 99135 6-phosphogluconate dehydrogenase 67046 3-methylglutaconic aciduria type 1 deficiency 238769 1q44 microdeletion syndrome 111 3-methylglutaconic aciduria type 2 13 6-pyruvoyl-tetrahydropterin synthase 976 2,8 dihydroxyadenine urolithiasis deficiency 67047 3-methylglutaconic aciduria type 3 869 2A syndrome 75857 6q terminal deletion 67048 3-methylglutaconic aciduria type 4 79154 2-aminoadipic 2-oxoadipic aciduria 171829 6q16 deletion syndrome 66634 3-methylglutaconic aciduria type 5 19 2-hydroxyglutaric acidemia 251056 6q25 microdeletion syndrome 352328 3-methylglutaconic -

Chediak‑Higashi Syndrome in Three Indian Siblings
Case Report Silvery Hair with Speckled Dyspigmentation: Chediak‑Higashi Access this article online Website: Syndrome in Three Indian Siblings www.ijtrichology.com Chekuri Raghuveer, Sambasiviah Chidambara Murthy, DOI: Mallur N Mithuna, Tamraparni Suresh 10.4103/0974-7753.167462 Quick Response Code: Department of Dermatology and Venereology, Vijayanagara Institute of Medical Sciences, Bellary, Karnataka, India ABSTRACT Silvery hair is a common feature of Chediak-Higashi syndrome (CHS), Griscelli syndrome, and Elejalde syndrome. CHS is a rare autosomal recessive disorder characterized by partial oculocutaneous albinism, frequent pyogenic infections, and the presence of abnormal large granules in leukocytes and other granule containing cells. A 6-year-old girl had recurrent Address for correspondence: respiratory infections, speckled hypo- and hyper-pigmentation over exposed areas, and Dr. Chekuri Raghuveer, silvery hair since early childhood. Clinical features, laboratory investigations, hair microscopy, Department of Dermatology and skin biopsy findings were consistent with CHS. Her younger sisters aged 4 and 2 years and Venereology, Vijayanagara had similar clinical, peripheral blood picture, and hair microscopy findings consistent with Institute of Medical Sciences, CHS. This case is reported for its rare occurrence in all the three siblings of the family, prominent pigmentary changes, and absent accelerated phase till date. Awareness, early Bellary ‑ 583 104, recognition, and management of the condition may prevent the preterm morbidity associated. Karnataka, India. E‑mail: c_raghuveer@ yahoo.com Key words: Partial albinism, primary immunodeficiency, silvery hair syndrome INTRODUCTION frontal scalp, eyebrows, eyelashes [Figure 1], and ocular pigmentary dilution was present. Other systems including hediak‑Higashi syndrome (CHS) is a rare, autosomal neurological findings were normal. -

Disorders of Pigmentation — Part 2: Hypopigmentation
How to Treat PULL-OUT SECTION www.australiandoctor.com.au COMPLETE HOW TO TREAT QUIZZES ONLINE www.australiandoctor.com.au/cpd to earn CPD or PDP points. INSIDE General approach Vitiligo Infective causes Other acquired disorders Genetic and congenital disorders THE AUTHORS DR DEEPANI RATHNAYAKE consultant dermatologist, Base Hospital Dambulla, Sri Lanka. Background DISORDERS of pigmentation are Disorders of PROFESSOR ROD SINCLAIR a common presentation in derma- director and head, dermatology, tology and general practice. Part 1 Epworth Hospital, Melbourne, of this update on disorders of pig- Victoria. mentation outlined the physiology of skin pigmentation and the path- pigmentation ological mechanisms that lead to these disorders. It then discussed the general approach to pigmentary dis- orders and focused on the diagnosis — Part 2: and management of hyperpigmen- tary disorders. Hypopigmentation, like hyper- pigmentation, can lead to signifi- cant psychosocial problems. It can Hypopigmentation even be a reason for social rejec- tion. This is especially pertinent in the cosmetic management of the This is the second of a two-part series on disorders of pigmentation. Part 2 covers hypopigmentary hypopigmented lesions that can be disorders in detail. significantly disfiguring on the face and other exposed skin. Acquired hypopigmentation may be associ- ated with an underlying systemic disorder that may be treatable. The search and treatment for underlying Copyright © 2014 conditions are just as important as Australian Doctor All rights reserved. No part of this the management of the presenting publication may be reproduced, cosmetic complaint. distributed, or transmitted in any This article discusses the general form or by any means without approach to hypopigmented skin the prior written permission of the publisher. -

(12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub
US 2010O2.10567A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2010/0210567 A1 Bevec (43) Pub. Date: Aug. 19, 2010 (54) USE OF ATUFTSINASATHERAPEUTIC Publication Classification AGENT (51) Int. Cl. A638/07 (2006.01) (76) Inventor: Dorian Bevec, Germering (DE) C07K 5/103 (2006.01) A6IP35/00 (2006.01) Correspondence Address: A6IPL/I6 (2006.01) WINSTEAD PC A6IP3L/20 (2006.01) i. 2O1 US (52) U.S. Cl. ........................................... 514/18: 530/330 9 (US) (57) ABSTRACT (21) Appl. No.: 12/677,311 The present invention is directed to the use of the peptide compound Thr-Lys-Pro-Arg-OH as a therapeutic agent for (22) PCT Filed: Sep. 9, 2008 the prophylaxis and/or treatment of cancer, autoimmune dis eases, fibrotic diseases, inflammatory diseases, neurodegen (86). PCT No.: PCT/EP2008/007470 erative diseases, infectious diseases, lung diseases, heart and vascular diseases and metabolic diseases. Moreover the S371 (c)(1), present invention relates to pharmaceutical compositions (2), (4) Date: Mar. 10, 2010 preferably inform of a lyophilisate or liquid buffersolution or artificial mother milk formulation or mother milk substitute (30) Foreign Application Priority Data containing the peptide Thr-Lys-Pro-Arg-OH optionally together with at least one pharmaceutically acceptable car Sep. 11, 2007 (EP) .................................. O7017754.8 rier, cryoprotectant, lyoprotectant, excipient and/or diluent. US 2010/0210567 A1 Aug. 19, 2010 USE OF ATUFTSNASATHERAPEUTIC ment of Hepatitis BVirus infection, diseases caused by Hepa AGENT titis B Virus infection, acute hepatitis, chronic hepatitis, full minant liver failure, liver cirrhosis, cancer associated with Hepatitis B Virus infection. 0001. The present invention is directed to the use of the Cancer, Tumors, Proliferative Diseases, Malignancies and peptide compound Thr-Lys-Pro-Arg-OH (Tuftsin) as a thera their Metastases peutic agent for the prophylaxis and/or treatment of cancer, 0008.