Silver in Drinking-Water
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Treatise on Combined Metalworking Techniques: Forged Elements and Chased Raised Shapes Bonnie Gallagher
Rochester Institute of Technology RIT Scholar Works Theses Thesis/Dissertation Collections 1972 Treatise on combined metalworking techniques: forged elements and chased raised shapes Bonnie Gallagher Follow this and additional works at: http://scholarworks.rit.edu/theses Recommended Citation Gallagher, Bonnie, "Treatise on combined metalworking techniques: forged elements and chased raised shapes" (1972). Thesis. Rochester Institute of Technology. Accessed from This Thesis is brought to you for free and open access by the Thesis/Dissertation Collections at RIT Scholar Works. It has been accepted for inclusion in Theses by an authorized administrator of RIT Scholar Works. For more information, please contact [email protected]. TREATISE ON COMBINED METALWORKING TECHNIQUES i FORGED ELEMENTS AND CHASED RAISED SHAPES TREATISE ON. COMBINED METALWORKING TECHNIQUES t FORGED ELEMENTS AND CHASED RAISED SHAPES BONNIE JEANNE GALLAGHER CANDIDATE FOR THE MASTER OF FINE ARTS IN THE COLLEGE OF FINE AND APPLIED ARTS OF THE ROCHESTER INSTITUTE OF TECHNOLOGY AUGUST ( 1972 ADVISOR: HANS CHRISTENSEN t " ^ <bV DEDICATION FORM MUST GIVE FORTH THE SPIRIT FORM IS THE MANNER IN WHICH THE SPIRIT IS EXPRESSED ELIEL SAARINAN IN MEMORY OF MY FATHER, WHO LONGED FOR HIS CHILDREN TO HAVE THE OPPORTUNITY TO HAVE THE EDUCATION HE NEVER HAD THE FORTUNE TO OBTAIN. vi PREFACE Although the processes of raising, forging, and chasing of metal have been covered in most technical books, to date there is no major source which deals with the functional and aesthetic requirements -

Dermatology Eponyms – Sign –Lexicon (P)
2XU'HUPDWRORJ\2QOLQH Historical Article Dermatology Eponyms – sign –Lexicon (P)� Part 2 Piotr Brzezin´ ski1,2, Masaru Tanaka3, Husein Husein-ElAhmed4, Marco Castori5, Fatou Barro/Traoré6, Satish Kashiram Punshi7, Anca Chiriac8,9 1Department of Dermatology, 6th Military Support Unit, Ustka, Poland, 2Institute of Biology and Environmental Protection, Department of Cosmetology, Pomeranian Academy, Slupsk, Poland, 3Department of Dermatology, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan, 4Department of Dermatology, San Cecilio University Hospital, Granada, Spain, 5Medical Genetics, Department of Experimental Medicine, Sapienza - University of Rome, San Camillo-Forlanini Hospital, Rome, Italy, 6Department of Dermatology-Venerology, Yalgado Ouédraogo Teaching Hospital Center (CHU-YO), Ouagadougou, Burkina Faso, 7Consultant in Skin Dieseases, VD, Leprosy & Leucoderma, Rajkamal Chowk, Amravati – 444 601, India, 8Department of Dermatology, Nicolina Medical Center, Iasi, Romania, 9Department of Dermato-Physiology, Apollonia University Iasi, Strada Muzicii nr 2, Iasi-700399, Romania Corresponding author: Piotr Brzezin′ski, MD PhD, E-mail: [email protected] ABSTRACT Eponyms are used almost daily in the clinical practice of dermatology. And yet, information about the person behind the eponyms is difficult to find. Indeed, who is? What is this person’s nationality? Is this person alive or dead? How can one find the paper in which this person first described the disease? Eponyms are used to describe not only disease, but also clinical signs, surgical procedures, staining techniques, pharmacological formulations, and even pieces of equipment. In this article we present the symptoms starting with (P) and other. The symptoms and their synonyms, and those who have described this symptom or phenomenon. Key words: Eponyms; Skin diseases; Sign; Phenomenon Port-Light Nose sign or tylosis palmoplantaris is widely related with the onset of squamous cell carcinoma of the esophagus. -

Repoussé Work for Amateurs
rf Bi oN? ^ ^ iTION av op OCT i 3 f943 2 MAY 8 1933 DEC 3 1938 MAY 6 id i 28 dec j o m? Digitized by the Internet Archive in 2011 with funding from Boston Public Library http://www.archive.org/details/repoussworkforamOOhasl GROUP OF LEAVES. Repousse Work for Amateurs. : REPOUSSE WORK FOR AMATEURS: BEING THE ART OF ORNAMENTING THIN METAL WITH RAISED FIGURES. tfjLd*- 6 By L. L. HASLOPE. ILLUSTRATED. LONDON L. UPCOTT GILL, 170, STRAND, W.C, 1887. PRINTED BY A. BRADLEY, 170, STRAND, LONDON. 3W PREFACE. " JjJjtfN these days, when of making books there is no end," ^*^ and every description of work, whether professional or amateur, has a literature of its own, it is strange that scarcely anything should have been written on the fascinating arts of Chasing and Repousse Work. It is true that a few articles have appeared in various periodicals on the subject, but with scarcely an exception they treated only of Working on Wood, and the directions given were generally crude and imperfect. This is the more surprising when we consider how fashionable Repousse Work has become of late years, both here and in America; indeed, in the latter country, "Do you pound brass ? " is said to be a very common question. I have written the following pages in the hope that they might, in some measure, supply a want, and prove of service to my brother amateurs. It has been hinted to me that some of my chapters are rather "advanced;" in other words, that I have gone farther than amateurs are likely to follow me. -

Guide to Policy & Practice Questions
OREGON BOARD OF CHIROPRACTIC EXAMINERS GUIDE TO POLICY & PRACTICE QUESTIONS 530 Center St NE, suite 620 Salem, OR 97301 (503) 378-5816 [email protected] Updated/Adopted: 9/17/2020 TABLE OF CONTENTS SECTION I ............................................................................................................................................................................................... 6 DEVICES, PROCEDURES, AND SUBSTANCES ............................................................................................................................... 6 DEVICES ................................................................................................................................................................ 6 BAX 3000 AND SIMILAR DEVICES................................................................................................................................................ 6 BIOPTRON LIGHT THERAPY ........................................................................................................................................................ 6 CPAP MACHINE, ORDERING ....................................................................................................................................................... 6 CTD MARK I MULTI-TORSION TRACTION DEVICE................................................................................................................... 6 DYNATRON 2000 ........................................................................................................................................................................... -

Silver Cas # 7440-22-4
SILVER CAS # 7440-22-4 Agency for Toxic Substances and Disease Registry ToxFAQs July 1999 This fact sheet answers the most frequently asked health questions (FAQs) about silver. For more information, call the ATSDR Information Center at 1-888-422-8737. This fact sheet is one in a series of summaries about hazardous substances and their health effects. It’s important you understand this information because this substance may harm you. The effects of exposure to any hazardous substance depend on the dose, the duration, how you are exposed, personal traits and habits, and whether other chemicals are present. HIGHLIGHTS: Silver is an element found naturally in the environment. At very high levels, it may cause argyria, a blue-gray discoloration of the skin and other organs. This chemical has been found in at least 27 of the 1,177 National Priorities List sites identified by the Environmental Protection Agency (EPA). What is silver? o It may be released into water from photographic process ing. (Pronounced s≥l vír) o Rain may wash silver out of soil into the groundwater. Silver is a naturally occurring element. It is found in the o Silver does not appear to concentrate to a significant environment combined with other elements such as sulfide, extent in aquatic animals. chloride, and nitrate. Pure silver is “silver” colored, but silver nitrate and silver chloride are powdery white and silver sul fide and silver oxide are dark-gray to black. Silver is often How might I be exposed to silver? found as a by-product during the retrieval of copper, lead, o Breathing low levels in air. -

Metals and Metal Products Tariff Schedules of the United States
251 SCHEDULE 6. - METALS AND METAL PRODUCTS TARIFF SCHEDULES OF THE UNITED STATES SCHEDULE 6. - METALS AND METAL PRODUCTS 252 Part 1 - Metal-Bearing Ores and Other Metal-Bearing Schedule 6 headnotes: Materials 1, This schedule does not cover — Part 2 Metals, Their Alloys, and Their Basic Shapes and Forms (II chemical elements (except thorium and uranium) and isotopes which are usefully radioactive (see A. Precious Metals part I3B of schedule 4); B. Iron or Steel (II) the alkali metals. I.e., cesium, lithium, potas C. Copper sium, rubidium, and sodium (see part 2A of sched D. Aluminum ule 4); or E. Nickel (lii) certain articles and parts thereof, of metal, F. Tin provided for in schedule 7 and elsewhere. G. Lead 2. For the purposes of the tariff schedules, unless the H. Zinc context requires otherwise — J. Beryllium, Columbium, Germanium, Hafnium, (a) the term "precious metal" embraces gold, silver, Indium, Magnesium, Molybdenum, Rhenium, platinum and other metals of the platinum group (iridium, Tantalum, Titanium, Tungsten, Uranium, osmium, palladium, rhodium, and ruthenium), and precious- and Zirconium metaI a Iloys; K, Other Base Metals (b) the term "base metal" embraces aluminum, antimony, arsenic, barium, beryllium, bismuth, boron, cadmium, calcium, chromium, cobalt, columbium, copper, gallium, germanium, Part 3 Metal Products hafnium, indium, iron, lead, magnesium, manganese, mercury, A. Metallic Containers molybdenum, nickel, rhenium, the rare-earth metals (Including B. Wire Cordage; Wire Screen, Netting and scandium and yttrium), selenium, silicon, strontium, tantalum, Fencing; Bale Ties tellurium, thallium, thorium, tin, titanium, tungsten, urani C. Metal Leaf and FoU; Metallics um, vanadium, zinc, and zirconium, and base-metal alloys; D, Nails, Screws, Bolts, and Other Fasteners; (c) the term "meta I" embraces precious metals, base Locks, Builders' Hardware; Furniture, metals, and their alloys; and Luggage, and Saddlery Hardware (d) in determining which of two or more equally specific provisions for articles "of iron or steel", "of copper", E. -

Clinicopathological Correlation of Acquired Hyperpigmentary Disorders
Symposium Clinicopathological correlation of acquired Dermatopathology hyperpigmentary disorders Anisha B. Patel, Raj Kubba1, Asha Kubba1 Department of Dermatology, ABSTRACT Oregon Health Sciences University, Portland, Oregon, Acquired pigmentary disorders are group of heterogenous entities that share single, most USA, 1Delhi Dermatology Group, Delhi Dermpath significant, clinical feature, that is, dyspigmentation. Asians and Indians, in particular, are mostly Laboratory, New Delhi, India affected. Although the classic morphologies and common treatment options of these conditions have been reviewed in the global dermatology literature, the value of histpathological evaluation Address for correspondence: has not been thoroughly explored. The importance of accurate diagnosis is emphasized here as Dr. Asha Kubba, the underlying diseases have varying etiologies that need to be addressed in order to effectively 10, Aradhana Enclave, treat the dyspigmentation. In this review, we describe and discuss the utility of histology in the R.K. Puram, Sector‑13, diagnostic work of hyperpigmentary disorders, and how, in many cases, it can lead to targeted New Delhi ‑ 110 066, India. E‑mail: and more effective therapy. We focus on the most common acquired pigmentary disorders [email protected] seen in Indian patients as well as a few uncommon diseases with distinctive histological traits. Facial melanoses, including mimickers of melasma, are thoroughly explored. These diseases include lichen planus pigmentosus, discoid lupus erythematosus, drug‑induced melanoses, hyperpigmentation due to exogenous substances, acanthosis nigricans, and macular amyloidosis. Key words: Facial melanoses, histology of hyperpigmentary disorders and melasma, pigmentary disorders INTRODUCTION focus on the most common acquired hyperpigmentary disorders seen in Indian patients as well as a few Acquired pigmentary disorders are found all over the uncommon diseases with distinctive histological traits. -

Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: a Review
antibiotics Review Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review Masaya Shimabukuro Department of Biomaterials, Faculty of Dental Science, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan; [email protected]; Tel.: +81-92-642-6346 Received: 3 October 2020; Accepted: 19 October 2020; Published: 20 October 2020 Abstract: Titanium (Ti) and its alloys are commonly used in medical devices. However, biomaterial-associated infections such as peri-implantitis and prosthetic joint infections are devastating and threatening complications for patients, dentists, and orthopedists and are easily developed on titanium surfaces. Therefore, this review focuses on the formation of biofilms on implant surfaces, which is the main cause of infections, and one-step micro-arc oxidation (MAO) as a coating technology that can be expected to prevent infections due to the implant. Many researchers have provided sufficient data to prove the efficacy of MAO for preventing the initial stages of biofilm formation on implant surfaces. Silver (Ag), copper (Cu), and zinc (Zn) are well used and are incorporated into the Ti surface by MAO. In this review, the antibacterial properties, cytotoxicity, and durability of these elements on the Ti surface incorporated by one-step MAO will be summarized. This review is aimed at enhancing the importance of the quantitative control of Ag, Cu, and Zn for their use in implant surfaces and the significance of the biodegradation behavior of these elements for the development of antibacterial properties. Keywords: titanium; biofilm; infection; micro-arc oxidation; silver; copper; zinc; antibacterial properties; coating; implant 1. -

Rhodium Products
Rhodium products Rhodium is one of the of six elements in the platinum group, which consists of platinum, palladium, rhodium, osmium, iridium and ruthenium. Often found with deposits of platinum and commonly obtained from the mining and refining of platinum, it is considered to be the rarest and most valuable precious metal, more valuable than gold or silver. Rhodium is a silver-white metallic element with high melting and boiling points. It is highly reflective and resistant to corrosion and oxidation, which is why it is also classified as a noble metal. It was discovered in 1803 by English chemist William Hyde Wollaston shortly after his discovery of palladium. Wollaston extracted rhodium from a piece of platinum ore that he had obtained from South America. Rhodium was named for the rose-red color of its salts, after the Greek word “rhodon” which means rose. Rarely used by itself, rhodium metal is almost always used as an alloy. We offer a broad, diverse catalog of rhodium products which are also available in bulk quantities and pack sizes that can be customized to your requirements. Application highlights: The Alfa Aesar™ portfolio of rhodium products can be used in a wide range of applications, from chemistry research to manufacturing and industry, from emission control and electrical applications to jewelry. Rhodium in chemistry Rhodium is used in research and industrial laboratories primarily as a catalyst. It is preferable to the other platinum group catalysts in the reduction of nitrogen oxides to nitrogen and oxygen. Rhodium is also used to catalyze the reduction of benzene to cyclohexane as well as the addition of hydrosilanes to double bonds, an important step in the manufacture of certain silicone rubbers. -

Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal
ytology & f C H o is Prakash, et al., J Cytol Histol 2018, 9:2 l t a o n l o r DOI: 10.4172/2157-7099.1000501 g u y o J Journal of Cytology & Histology ISSN: 2157-7099 Research Article Open Access Multi-Organ Teratogenesis Sequels of Bigger Size Particles Colloidal Silver in Primate Vertebrates Pani Jyoti Prakash*1, Singh Royana2 and Pani Sankarsan3 1Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India 2Department of Anatomy, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttarpradesh, India 3Deapartment of Surgery, Institute of Medical Science and Research, Karjat, Bhivpuri, India *Corresponding author: Prakash PJ, Department of Anatomy, Faculty of Medicine, Institute of Medical Science and Research, Karjat, Bhivpuri, India, Tel: 8433668356; E-mail: [email protected] Received date: February 21, 2018; Accepted date: March 12, 2018; Published date: March 16, 2018 Copyright: © 2018 Prakash PJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Back ground: In this most recent, update global arena for consumers products most of the daily applications of bigger silver nano particles (20 to 100 nano meter range) are effected as anti-viral and anti-parasitic agents in clinical medicine and diagnosis which is a positive feedback. However, the major negative feedback of bigger size silver nano particles on human, animal and primate vertebrate body is multisystem teratogenicity focuses. Material and methods: This study was designed to investigate teratogenic effects of bigger size nano silver which is poly vinyl pyrollidone coated and sodium borohydride stabilized. -

A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient with Comorbid Schizoaffective Disorder
A Case of Argyria: Multiple Forms of Silver Ingestion in a Patient With Comorbid Schizoaffective Disorder Sarah J. Schrauben, MD; Dhaval G. Bhanusali, MD; Stuart Sheets, MD; Animesh A. Sinha, MD, PhD Argyria is a rare cutaneous manifestation of silver is an elemental compound often found in drinking deposits in the skin, characterized by a grayish water, various sources in industry, and alternative blue discoloration, particularly in sun-exposed medicine compounds. Silver was first used medically areas. We report the case of a patient with a his- in 980 ad; it was believed to provide a means of tory of schizoaffective disorder and type 2 diabe- blood purification, to treat heart palpitations, and to tes mellitus who presented with argyria of the face serve as an adjunct treatment of epilepsy and tabes and neck. The patient had a history of ingesting dorsalis.1 In the 1900s, silver began being used as a colloidal silver proteins (CSPs) for approximately popular treatment of infections. However, reports of 10 years as a self-prescribed remedy for his medi- unsolicited side effects diminished its popularity as a cal conditions. CUTISmainstay solution for many ailments. Silver recently Colloidal silver protein has gained popularity has regained popularity, with an increase in Internet among patients who seek alternative medical ther- claims promoting the use of oral colloidal silver pro- apies. Argyria is the most predominant manifesta- teins (CSPs) as mineral supplements and as a way to tion of silver toxicity. It is unclear if our patient prevent and treat many diseases.2 began taking CSP becauseDo of his schizoaffectiveNot WeCopy present a patient with argyria as a conse- disorder or if silver toxicity may have induced quence of multiple forms of silver ingestion in an somatic delusions; however, it is important for attempt to treat his type 2 diabetes mellitus and physicians to have a thorough understanding of schizoaffective disorder. -
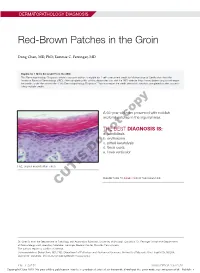
Red-Brown Patches in the Groin
DERMATOPATHOLOGY DIAGNOSIS Red-Brown Patches in the Groin Dong Chen, MD, PhD; Tammie C. Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 66-year-old man presented with reddish arciform patchescopy in the inguinal area. THE BEST DIAGNOSIS IS: a. candidiasis b. noterythrasma c. pitted keratolysis d. tinea cruris Doe. tinea versicolor H&E, original magnification ×600. PLEASE TURN TO PAGE 419 FOR THE DIAGNOSIS CUTIS Dr. Chen is from the Department of Pathology and Anatomical Sciences, University of Missouri, Columbia. Dr. Ferringer is from the Departments of Dermatology and Laboratory Medicine, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Dong Chen, MD, PhD, Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Dr, MA204, DC018.00, Columbia, MO 65212 ([email protected]). 416 I CUTIS® WWW.MDEDGE.COM/CUTIS Copyright Cutis 2018. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Erythrasma rythrasma usually involves intertriginous areas surface (Figure 1) compared to dermatophyte hyphae that (eg, axillae, groin, inframammary area). Patients tend to be parallel to the surface.2 E present with well-demarcated, minimally scaly, red- Pitted keratolysis is a superficial bacterial infection brown patches.