Eczema Vaccinatum: Report of Two Cases with a Review of the Literature'
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Poxviruses: Smallpox Vaccine, Its Complications and Chemotherapy
Virus Adaptation and Treatment Dovepress open access to scientific and medical research Open Access Full Text Article R E V IEW Poxviruses: smallpox vaccine, its complications and chemotherapy Mimi Remichkova Abstract: The threat of bioterrorism in the recent years has once again posed to mankind the unresolved problems of contagious diseases, well forgotten in the past. Smallpox (variola) is Department of Pathogenic Bacteria, The Stephan Angeloff Institute among the most dangerous and highly contagious viral infections affecting humans. The last of Microbiology, Bulgarian Academy natural case in Somalia marked the end of a successful World Health Organization campaign of Sciences, Sofia, Bulgaria for smallpox eradication by vaccination on worldwide scale. Smallpox virus still exists today in some laboratories, specially designated for that purpose. The contemporary response in the treatment of the post-vaccine complications, which would occur upon enforcing new programs for mass-scale smallpox immunization, includes application of effective chemotherapeutics and their combinations. The goals are to provide the highest possible level of protection and safety of For personal use only. the population in case of eventual terrorist attack. This review describes the characteristic features of the poxviruses, smallpox vaccination, its adverse reactions, and poxvirus chemotherapy. Keywords: poxvirus, smallpox vaccine, post vaccine complications, inhibitors Characteristics of poxviruses Smallpox (variola) infection is caused by the smallpox virus. This virus belongs to the genus of Orthopoxvirus included in the Poxviridae family. Poxviruses are one of the largest and most complexly structured viruses, known so far. The genome of poxviruses consists of a linear two-chained DNA and its replication takes place in the cytoplasm of the infected cell. -
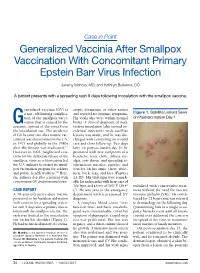
Generalized Vaccinia After Smallpox Vaccination with Concomitant Primary Epstein Barr Virus Infection
Case in Point Generalized Vaccinia After Smallpox Vaccination With Concomitant Primary Epstein Barr Virus Infection Jeremy Mandia, MD; and Kathryn Buikema, DO A patient presents with a spreading rash 9 days following inoculation with the smallpox vaccine. eneralized vaccinia (GV) is atopic dermatitis, or other rashes a rare, self-limiting complica- and reported no systemic symptoms. Figure 1. Satellite Lesions Seen tion of the smallpox vacci- His vitals also were within normal on Postvaccination Day 1 Gnation that is caused by the limits. A clinical diagnosis of inad- systemic spread of the virus from vertent inoculation (also termed ac- the inoculation site. The incidence cidental infection) with satellite of GV became rare after routine vac- lesions was made, and he was dis- cination was discontinued in the U.S. charged with counseling on wound in 1971 and globally in the 1980s care and close follow-up. Two days after the disease was eradicated.1,2 later, on postvaccination day 11, he However in 2002, heightened con- presented with new symptoms of a cerns for the deliberate release of the headache, fever, chills, diffuse my- smallpox virus as a bioweapon led algia, sore throat, and spreading er- the U.S. military to restart its small- ythematous macules, papules, and pox vaccination program for soldiers vesicles on his arms, chest, abdo- and public health workers.3,4 Here, men, back, legs, and face (Figures the authors describe a patient with 2A-2D). His vital signs were remark- concomitant GV and mononucleosis. able for tachycardia with heart rate of 100 bpm and a fever of 103º F (39.4º stabilized with conservative treat- CASE REPORT C). -

Medical Management of Biological Casualties Handbook
USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 U.S. ARMY MEDICAL RESEARCH INSTITUTE OF INFECTIOUS DISEASES FORT DETRICK FREDERICK, MARYLAND Emergency Response Numbers National Response Center: 1-800-424-8802 or (for chem/bio hazards & terrorist events) 1-202-267-2675 National Domestic Preparedness Office: 1-202-324-9025 (for civilian use) Domestic Preparedness Chem/Bio Helpline: 1-410-436-4484 or (Edgewood Ops Center – for military use) DSN 584-4484 USAMRIID’s Emergency Response Line: 1-888-872-7443 CDC'S Emergency Response Line: 1-770-488-7100 Handbook Download Site An Adobe Acrobat Reader (pdf file) version of this handbook can be downloaded from the internet at the following url: http://www.usamriid.army.mil USAMRIID’s MEDICAL MANAGEMENT OF BIOLOGICAL CASUALTIES HANDBOOK Sixth Edition April 2005 Lead Editor Lt Col Jon B. Woods, MC, USAF Contributing Editors CAPT Robert G. Darling, MC, USN LTC Zygmunt F. Dembek, MS, USAR Lt Col Bridget K. Carr, MSC, USAF COL Ted J. Cieslak, MC, USA LCDR James V. Lawler, MC, USN MAJ Anthony C. Littrell, MC, USA LTC Mark G. Kortepeter, MC, USA LTC Nelson W. Rebert, MS, USA LTC Scott A. Stanek, MC, USA COL James W. Martin, MC, USA Comments and suggestions are appreciated and should be addressed to: Operational Medicine Department Attn: MCMR-UIM-O U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID) Fort Detrick, Maryland 21702-5011 PREFACE TO THE SIXTH EDITION The Medical Management of Biological Casualties Handbook, which has become affectionately known as the "Blue Book," has been enormously successful - far beyond our expectations. -

Vaccinia Virus
APPENDIX 2 Vaccinia Virus • Accidental infection following transfer from the vac- cination site to another site (autoinoculation) or to Disease Agent: another person following intimate contact Likelihood of Secondary Transmission: • Vaccinia virus • Significant following direct contact Disease Agent Characteristics: At-Risk Populations: • Family: Poxviridae; Subfamily: Chordopoxvirinae; • Individuals receiving smallpox (vaccinia) vaccination Genus: Orthopoxvirus • Individuals who come in direct contact with vacci- • Virion morphology and size: Enveloped, biconcave nated persons core with two lateral bodies, brick-shaped to pleo- • Those at risk for more severe complications of infec- morphic virions, ~360 ¥ 270 ¥ 250 nm in size tion include the following: • Nucleic acid: Nonsegmented, linear, covalently ᭺ Immune-compromised persons including preg- closed, double-stranded DNA, 18.9-20.0 kb in length nant women • Physicochemical properties: Virus is inactivated at ᭺ Patients with atopy, especially those with eczema 60°C for 8 minutes, but antigen can withstand 100°C; ᭺ Patients with extensive exfoliative skin disease lyophilized virus maintains potency for 18 months at 4-6°C; virus may be stable when dried onto inanimate Vector and Reservoir Involved: surfaces; susceptible to 1% sodium hypochlorite, • No natural host 2% glutaraldehyde, and formaldehyde; disinfection of hands and environmental contamination with soap Blood Phase: and water are effective • Vaccinia DNA was detected by PCR in the blood in 6.5% of 77 military members from 1 to 3 weeks after Disease Name: smallpox (vaccinia) vaccination that resulted in a major skin reaction. • Progressive vaccinia (vaccinia necrosum or vaccinia • In the absence of complications after immunization, gangrenosum) recently published PCR and culture data suggest that • Generalized vaccinia viremia with current vaccines must be rare 3 weeks • Eczema vaccinatum after vaccination. -

Draft Recommendations for Use of Smallpox Vaccine in a Pre-Event
Draft Recommendations for Use of Smallpox Vaccine in a Pre-Event Smallpox Vaccination Program: Supplemental Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC) In June 2001, the Advisory Committee on Immunization Practices (ACIP) made recommendations for the use of smallpox (vaccinia) vaccine to protect persons working with orthopoxviruses, and to prepare for a possible bioterrorism attack and for response to an attack involving smallpox.[1] Because of the terrorist attacks in the fall of 2001, the Centers for Disease Control and Prevention (CDC) asked the ACIP to review their previous recommendations for smallpox vaccination. These supplemental recommendations update the 2001 recommendations for vaccination of persons designated to respond or care for a suspected or confirmed case of smallpox. In addition, they clarify and expand the primary strategy for control and containment of smallpox in the event of an outbreak. Recommendations for vaccination of laboratory workers who directly handle recombinant vaccinia viruses derived from non-highly attenuated vaccinia strains, or other orthopoxviruses that infect humans (e.g., monkeypox, cowpox, vaccinia, and variola) remain unchanged.[1] The following recommendations were developed after formation of a joint Working Group of the ACIP and the National Vaccine Advisory Committee (NVAC) in April 2002, joined in September 2002 by the Healthcare Infection Control Practices Advisory Committee (HICPAC), and a series of public meetings and forums to review available data on smallpox, smallpox vaccine, smallpox control strategies, and other issues related to smallpox vaccination. Smallpox Transmission and Control Smallpox is transmitted from an infected person. Patients are most infectious during the first 7 to 10 days following rash onset; transmission can occur during the prodromal period just prior to rash onset, when lesions in the mouth ulcerate, releasing virus into oral secretions. -

Painful Bubbles
Osteopathic Family Physician (2018) 29 - 31 29 CLINICAL IMAGES Painful Bubbles Craig Bober, DO & Amy Schultz, DO Lankenau Hospital Family Medicine Residency A 25 year-old female with a past medical history of well controlled eczema presented to her primary care physician with a one week his- tory of a painful “bubbles” localized to her right antecubital fossa as seen in Figure 1. She noted that the new rash appeared to form over- night, was extremely painful, and would occasionally drain a clear liquid after scratching. It did not respond to her usual over-the-counter regimen of moisturizers prompting her to be evaluated. She had subjective fevers and malaise but denied oral or genital ulcers, vaginal discharge, dysuria, ocular irritation, visual disturbances, and upper respiratory or gastrointestinal symptoms. Review of systems was oth- erwise unremarkable. She had no other known medical problems, allergies, and denied drug and alcohol use. She denied any recent travel, sick contacts, pets, or OTC medications/creams. She was sexually active in a monogamous relationship for over a year. QUESTIONS 1. What is the most likely diagnosis? A. Cellulitis B. Eczema herpeticum C. Impetigo D. Primary varicella infection 2. Which test should be performed initally? A. Blood culture B. Direct fuorescent antibody staining FIGURE 1: C. Tzanck smear D. Wound culture 3. What is the best treatment? A. Acyclovir B. Augmentin C. Doxycycline D. Varicella Zoster Immune Globulin CORRESPONDENCE: Amy Schultz, DO | [email protected] 1877-5773X/$ - see front matter. © 2018 ACOFP. All rights reserved. 30 Osteopathic Family Physician | Volume 10, No. 3 | May/June, 2018 ANSWERS 1. -

Eczema Vaccinatum in Indiana’S Public Health Nurses Play a Vital Role in Protecting, Aiding, Child Resulting from and Educating Hoosiers
Volume 10, Issue 6 June 2007 Public Health Nurse Conference Article Page 2007 No. Public Health Nurse Tom Duszynski, BS Conference 2007 1 Eczema Vaccinatum in Indiana’s public health nurses play a vital role in protecting, aiding, Child Resulting from and educating Hoosiers. The Indiana State Department of Health Transmission of (ISDH) recognizes the contribution these nurses make to public health Vaccinia from in Indiana and assists their efforts by offering continuing education Smallpox Vaccinee opportunities, such as the annual Public Health Nurse Conference. with Tertiary Spread to the Mother 3 On June 8, more than 150 public A Decade of Indiana health nurses and Sentinel Influenza Data nursing students Surveillance 8 attended the 2007 ISDH Public Training Room 13 Health Nurse Conference. This Data Reports 14 year’s conference was the most well HIV Summary 14 attended in conference history. Disease Reports 15 Several years ago, the conference began as a “training day” to provide newly hired public health nurses with general education about public health responsibilities within local health departments. The conference has consistently grown over the past several years, and new ideas and different topics have emerged based on suggestions from those who have attended. Conference planners have used participants’ input to restructure the program to meet the needs of public health nurses. This year’s conference was no exception. Deputy State Health Commissioner Mary Hill, an attorney and registered nurse, opened the conference by reminding public health nurses and nursing students of the important work they do every day for Hoosiers and how, since September 11, 2001, their roles and knowledge have changed and expanded to include the world of preparedness, again demonstrating the flexibility of public health nursing. -

(12) Patent Application Publication (10) Pub. No.: US 2009/0221523 A1 Tseng Et Al
US 2009.022 1523A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0221523 A1 Tseng et al. (43) Pub. Date: Sep. 3, 2009 (54) NORTH-2'-DEOXY-METHANO- (86). PCT No.: PCT/US2O06/02O894 CARBATHYMIDNES AS ANTIVIRAL AGENTS AGAINST POXVIRUSES S371 (c)(1), (2), (4) Date: Apr. 3, 2009 (76) Inventors: Christopher K. Tseng, Related U.S. Application Data Burtonsville, MD (US); Victor E. (60) Provisional application No. 60/684.811, filed on May Marquez, Montgomery Village, 25, 2005. MD (US) Publication Classification (51) Int. Cl. Correspondence Address: A 6LX 3L/7072 (2006.01) KNOBBE, MARTENS, OLSON & BEAR, LLP A6IP3L/20 (2006.01) 2040 MAINSTREET, FOURTEENTH FLOOR (52) U.S. Cl. .......................................................... S14/SO IRVINE, CA 92.614 (US) (57) ABSTRACT (21) Appl. No.: 11/920,881 A method for the prevention or treatment of poxvirus infec tion by administering an effective amount of an antiviral agent comprising cyclopropanated carbocyclic 2'-deoxy (22) PCT Filed: May 25, 2006 nucleoside to an individual in need thereof is provided. Patent Application Publication Sep. 3, 2009 Sheet 2 of 3 US 2009/022 1523 A1 HSV-2 TK Goalpox TK humonl TK VV TK VOriod TK monkeypox TK fowlpox TK HSV-1 K african swine fever virus TK VZV TK EBV TK FIF2 Patent Application Publication Sep. 3, 2009 Sheet 3 of 3 US 2009/022 1523 A1 CC (N)-MCT CDV VC – -2 48.61 site - 2322 2027 . US 2009/022 1523 A1 Sep. 3, 2009 NORTH-2-DEOXY. breaks depended on the isolation of infected individuals and METHANOCARBATHYMIDNES AS the vaccination of close contacts. -

Delivery Information Form Cord Blood Program Header for PSBC Use Only NMDP BBCS Donor ID______CBU Transportation Box #______ID
Delivery Information Form Cord Blood Program Header for PSBC use only NMDP BBCS Donor ID________________________ CBU Transportation Box #____________________ ID: Emp ID___________ Date_______________ Emp ID___________ Date_______________ Virology NMDP HPC, Cord Blood Local DIN: Samples Maternal DIN: ID: Emp ID___________ Date_______________ Emp ID___________ Date_____________________ MATERNAL INFORMATION Apply pre-printed hospital label or fill in Mother’s Full Name: Mother’s Medical Record Number: Mother’s Date of Birth: COLLECTION INFORMATION Name of Hospital where Delivery Occurred: Approximate Gestational Age (must be ≥ 37 weeks): _ weeks Approximate Date and Time of membrane rupture: Date: _______________ Time: ______________ Infant’s Date and Time of Birth: Date: _______________ Time: ______________ Infant’s Sex: Female Male Cord Blood Unit Collection Date and Time: Date: _______________ Time: ______________ Type of Delivery: Vaginal C-section DELIVERY INFORMATION See back of form for a list of relevant complications/abnormalities and additional guidelines for physical assessment of the donor baby and mother. Add comments below as needed. Regardless of answer, the donor may still donate. Were there any abnormalities observed in the baby and/or complications of birth/pregnancy that could affect the cord blood? Yes No Were any findings detected on the physical exam of the donor mother that may indicate risk behavior for or infection with a communicable disease? Yes No VERIFICATION A trained cord blood collector followed the cord blood collection instructions included in the collection kit, confirmed that physical assessments were done on the donor mother and baby, and verified that the patient’s identity matches the identity on the cord blood unit and paperwork, and that all labeling and paperwork are legible. -

Structural and Functional Analysis of the Vaccinia Virus O1 Virulence Protein
Structural and functional analysis of the Vaccinia virus O1 virulence protein by Anastasia C. Weeks June 2017 Director of Thesis: Mark Mannie, Ph.D. Major Department: Biomedical Sciences, Microbiology and Immunology Poxviruses are double-stranded DNA viruses capable of causing disfiguring and deadly disease in a wide range of hosts, from insects to mammals. Orthopoxviruses (OPXV) encode many proteins that are not essential for viral replication, but are responsible for vast differences in pathogenesis. Of the >200 proteins in the prototypical OPXV vaccinia virus (VACV), many remain functionally cryptic. The objective of these studies was to understand how the VACV O1 protein functions by investigating cell-specific effects that may contribute to virulence. The O1L gene is expressed early as the O1 protein, a 78 kDa protein that lacked N-linked glycosylation. These data are the first to demonstrate the reduced ability of an O1 deletion mutant (∆O1) to induce cell migration compared to the parental VACV Western Reserve strain (VACV- WR). ∆O1-infected cell monolayers also exhibited reduced plaque diameter and clearance in plaque foci. These observations indicated that O1 is a significant contributor to VACV cytopathic effects (CPE) in vitro, in agreement with published reports. The results reported herein are the first to describe an altered immunological response with ∆O1, as levels of anti-VACV immunoglobulin significantly increased with ∆O1 infection at a time point (seven days post-infection) when VACV- WR induced VACV-specific antibody levels were comparable to sera from mock-infected mice. ∆O1 was more immunogenic in an ex vivo antigen presentation assay, although mitogen-induced CD4+ T cell activation during ∆O1 infection was equivalent to VACV-WR infection. -

Disseminated Varicella Zoster Virus Infection After Vaccination with a Live Attenuated Vaccine
PRACTICE | CASES SEPSIS CPD Disseminated varicella zoster virus infection after vaccination with a live attenuated vaccine Vinita Dubey MD, Derek MacFadden MD n Cite as: CMAJ 2019 September 16;191:E1025-7. doi: 10.1503/cmaj.190270 70-year-old man presented to the emergency depart- ment with a 2-week history of rash, which started as a KEY POINTS localized eruption on his forehead and progressed to a • Live attenuated vaccines are capable of causing symptomatic vesicularA rash involving his entire body (Figure 1). Over this same vaccine-derived infection, even weeks following vaccination. period, he noted increasing shortness of breath, tiredness, pain- • Immunocompromised hosts, including those taking low-dose ful swallowing and chills. He did not report recent travel. immunosuppressive medications, are at increased risk for The patient had a past history of hypertension, coronary artery infection with live vaccine strains. disease, congestive heart failure, chronic obstructive pulmonary • Caution is required before using live attenuated vaccines in immunocompromised people; expert consultation may be disease and atrial flutter. Successful cardiac ablation had been required. performed 2 weeks before the onset of the rash. He also had rheu- • Severe and unusual adverse events following vaccination matoid arthritis, treated with methotrexate, 2.5 mg/d (6 d per should be reported to local public health authorities for week) for 3 years, hydroxychloroquine, 200 mg/d and prednisone, surveillance and investigative purposes. 10 mg/d. In the previous month, his prednisone dosage had been tapered from 10 mg/d. He was not receiving any biologic agents. On examination, the patient’s blood pressure was 110/60 mm Hg, multiple vesicular and crusted lesions, disseminated varicella heart rate 86 beats/min and temperature 36.8°C. -

Skin Conditions That Mean You Should Not Get Smallpox Vaccine
SMALLPOX VACCINE INFORMATION STATEMENT (VIS) SUPPLEMENT C Skin Conditions That Mean You Should Not Get Smallpox Vaccine The smallpox vaccine is made from a live virus related to smallpox called vaccinia (not smallpox virus). The vaccine stimulates the immune system to react against the vaccinia virus, and develop immunity to it. Immunity to vaccinia also provides immunity to smallpox. For most people, live virus vaccines are safe and effective. However, people with certain skin conditions are more likely to have rare and serious reactions to the smallpox vaccine, including bad skin rashes (eczema vaccinatum).This results when virus from the vaccine site gets into broken skin and causes a rash in that area. While most people recover from this rash with treatment, it can be quite severe, sometimes leading to scarring or even death. SKIN CONDITIONS THAT MEAN YOU SHOULD NOT BE VACCINATED: • Individuals who have ever been diagnosed with eczema or atopic dermatitis, (conditions involving repeated episodes of red, itchy or inflamed skin) even if the condition is mild, not presently active, or if you had it only as a child, should not get the vaccine. • Individuals with Darier’s disease should not get the vaccine. • Individuals in close contact with someone who has ever been diagnosed with eczema or atopic dermatitis, even if the condition is mild, not presently active, or if they had it only as a child, should not get the vaccine because of the risk it poses to that close contact. (Close contacts include anyone living in your household and anyone you have close physical contact with such as a sexual partner.) SKIN CONDITIONS THAT MEAN YOU SHOULD WAIT BEFORE BEING VACCINATED: • Individuals with breaks in their skin should not be vaccinated until the skin is fully healed.