Psychiatric Disorders – Worldwide Advances
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
Pediatrics-EOR-Outline.Pdf
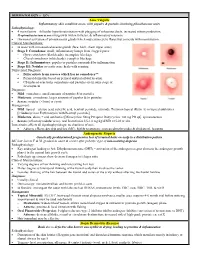
DERMATOLOGY – 15% Acne Vulgaris Inflammatory skin condition assoc. with papules & pustules involving pilosebaceous units Pathophysiology: • 4 main factors – follicular hyperkeratinization with plugging of sebaceous ducts, increased sebum production, Propionibacterium acnes overgrowth within follicles, & inflammatory response • Hormonal activation of pilosebaceous glands which may cause cyclic flares that coincide with menstruation Clinical Manifestations: • In areas with increased sebaceous glands (face, back, chest, upper arms) • Stage I: Comedones: small, inflammatory bumps from clogged pores - Open comedones (blackheads): incomplete blockage - Closed comedones (whiteheads): complete blockage • Stage II: Inflammatory: papules or pustules surrounded by inflammation • Stage III: Nodular or cystic acne: heals with scarring Differential Diagnosis: • Differentiate from rosacea which has no comedones** • Perioral dermatitis based on perioral and periorbital location • CS-induced acne lacks comedones and pustules are in same stage of development Diagnosis: • Mild: comedones, small amounts of papules &/or pustules • Moderate: comedones, larger amounts of papules &/or pustules • Severe: nodular (>5mm) or cystic Management: • Mild: topical – azelaic acid, salicylic acid, benzoyl peroxide, retinoids, Tretinoin topical (Retin A) or topical antibiotics [Clindamycin or Erythromycin with Benzoyl peroxide] • Moderate: above + oral antibiotics [Minocycline 50mg PO qd or Doxycycline 100 mg PO qd], spironolactone • Severe (refractory nodular acne): oral -

A ABCD, 19, 142, 152 ABCDE, 19, 142 Abramowitz Sign, 3, 5
Index A Atopic dermatitis, 2, 18, 19, 20, 30, ABCD, 19, 142, 152 61, 81, 82, 84, 86, 99 ABCDE, 19, 142 Atrophie blanche, 173, 177 Abramowitz sign, 3, 5 Atrophy, crinkling, 143, 160 Acanthosis nigricans, 105, 106, 132 Atypical nevus, 144, 145, 163, 164 Addison disease (primary adrenal eclipse, 144, 164 insufficiency), 137 ugly duckling, 144, 163 Albright (McCune–Albright Auspitz sign, 3, 5, 6, 18, 104 syndrome), 83, 93 All in different stages, 33, 34, 40 All in same stage, 33, 34, 38 B Alopecia, 57, 77, 94, 105, 106, 111, Bamboo hair, 84, 99, 171, 172 117, 119, 127, 133, 171, 172, Basal cell carcinoma, 17, 104, 140, 182, 184 151, 162, 170 Alopecia areata, 105, 106, 127, 171 Bioterrorism, 33, 38 Amyloid, 107, 119 Black dot (tinea capitis), 37, 57 Angiofibroma, 89 Blue angel (see Tumors, painful) Angiokeratoma, 3, 79, 81, 85 Blue rubber bleb nevus, 144, Angiolipoma, 142, 155 154, 168 Angioma, spider, 107, 109, 134 angiolipioma, 142, 155 Angiomatosis, leptomeningeal, 90 neurilemmoma, 142, 156 Anticoagulant, lupus, 108, 121 glomus tumor, 142, 157 Antiphospholipid syndrome eccrine spiradenoma, 142, 158 (APLS), 19, 107, 108, 121 leiomyoma, 142, 159 Apocrine hidrocystoma, 144, Blue cyst (apocrine hidrocystoma), 166, 168 144, 166, 168 Apple jelly, 36, 37, 47 Blue nose (purpura fulminans), 19, Ash leaf macule (confetti macule), 108, 122 82, 89 Blue papule (blue nevus), 1, 144, Asymmetry, 19, 118, 136, 152 166, 167, 168 187 188 Index Blue rubber bleb nevus, 144, 154, 168 Coast of California, 82, 83, 91 Border Coast of Maine, 83, 93 irregular, 83, -

Dermatological Indications of Disease - Part II This Patient on Dialysis Is Showing: A
“Cutaneous Manifestations of Disease” ACOI - Las Vegas FR Darrow, DO, MACOI Burrell College of Osteopathic Medicine This 56 year old man has a history of headaches, jaw claudication and recent onset of blindness in his left eye. Sed rate is 110. He has: A. Ergot poisoning. B. Cholesterol emboli. C. Temporal arteritis. D. Scleroderma. E. Mucormycosis. Varicella associated. GCA complex = Cranial arteritis; Aortic arch syndrome; Fever/wasting syndrome (FUO); Polymyalgia rheumatica. This patient missed his vaccine due at age: A. 45 B. 50 C. 55 D. 60 E. 65 He must see a (an): A. neurologist. B. opthalmologist. C. cardiologist. D. gastroenterologist. E. surgeon. Medscape This 60 y/o male patient would most likely have which of the following as a pathogen? A. Pseudomonas B. Group B streptococcus* C. Listeria D. Pneumococcus E. Staphylococcus epidermidis This skin condition, erysipelas, may rarely lead to septicemia, thrombophlebitis, septic arthritis, osteomyelitis, and endocarditis. Involves the lymphatics with scarring and chronic lymphedema. *more likely pyogenes/beta hemolytic Streptococcus This patient is susceptible to: A. psoriasis. B. rheumatic fever. C. vasculitis. D. Celiac disease E. membranoproliferative glomerulonephritis. Also susceptible to PSGN and scarlet fever and reactive arthritis. Culture if MRSA suspected. This patient has antithyroid antibodies. This is: • A. alopecia areata. • B. psoriasis. • C. tinea. • D. lichen planus. • E. syphilis. Search for Hashimoto’s or Addison’s or other B8, Q2, Q3, DRB1, DR3, DR4, DR8 diseases. This patient who works in the electronics industry presents with paresthesias, abdominal pain, fingernail changes, and the below findings. He may well have poisoning from : A. lead. B. -

Dermatology Grand Rounds 2019 Skin Signs of Internal Disease
Dermatology Grand Rounds 2019 skin signs of internal disease John Strasswimmer, MD, PhD Affiliate Clinical Professor (Dermatology), FAU College of Medicine Research Professor of Biochemistry, FAU College of Science Associate Clinical Professor, U. Miami Miller School of Medicine Dermatologist and Internal Medicine “Normal” abnormal skin findings in internal disease • Thyroid • Renal insufficiency • Diabetes “Abnormal” skin findings as clue to internal disease • Markers of infectious disease • Markers of internal malignancy risk “Consultation Cases” • Very large dermatology finding • A very tiny dermatology finding Dermatologist and Internal Medicine The "Red and Scaly” patient “Big and Small” red rashes not to miss The "Red and Scaly” patient • 29 Year old man with two year pruritic eruption • PMHx: • seasonal allergies • childhood eczema • no medications Erythroderma Erythroderma • Also called “exfoliative dermatitis” • Not stevens-Johnson / toxic epidermal necrosis ( More sudden onset, associated with target lesions, mucosal) • Generalized erythema and scale >80-90% of body surface • May be associated with telogen effluvium It is not a diagnosis per se Erythroderma Erythroderma Work up 1) Exam for pertinent positives and negatives: • lymphadenopathy • primary skin lesions (i.e. nail pits of psoriasis) • mucosal involvement • Hepatosplenomagaly 2) laboratory • Chem 7, LFT, CBC • HIV • Multiple biopsies over time 3) review of medications 4) age-appropriate malignancy screening 5) evaluate hemodynamic stability Erythroderma Management 1) -

Comparative Study of Halobetasol and Tacrolimus in Treatment of Lichen Planus
COMPARATIVE STUDY OF HALOBETASOL AND TACROLIMUS IN TREATMENT OF LICHEN PLANUS Dissertation Submitted in fulfillment of the university regulations for MD DEGREE IN DERMATOLOGY, VENEREOLOGY AND LEPROSY (BRANCH XII A) Madras Medical College Chennai THE TAMILNADU DR.M.G.R.MEDICAL UNIVERSITY CHENNAI APRIL 2011 CERTIFICATE Certified that this dissertation entitled “COMPARATIVE STUDY OF HALOBETASOL AND TACROLIMUS IN TREATMENT OF LICHEN PLANUS ” is a bonafide work done by Dr.VIDHYA RAVINDRAN, Post Graduate Student of the Department of Dermatology, Venereology and Leprosy, Madras Medical College, Chennai – 600 003, during the academic year 2008 – 2011. This work has not previously formed the basis for the award of any degree. Prof.Dr.D.PRABHAVATHY, MD.DD, Professor and Head of the Department, Department of Dermatology and Leprology, Madras Medical College, Chennai-600003. Prof. Dr. J.MOHANASUNDARAM, M.D.Ph.D, DNB., Dean, Madras Medical College, Chennai-600003 SPECIAL ACKNOWLEDGEMENT My sincere thanks to Prof. Dr.J.MOHANASUNDARAM, M.D ., PhD,DNB Dean, Madras Medical College for allowing me to do this dissertation and utilize the institutional facilities. ACKNOWLEDGEMENT This study which has been an integral part of my stay in the Department of Dermatology, Madras Medical College, Chennai wouldn’t have been completed without the help of great number of people. It gives me great pleasure in thanking them in the beginning. In the first place, I express my sincere gratitude to Prof. Dr.D.PRABHAVATHY, M.D.,D.D., Professor and Head, Department of Dermatology and Leprology and my co-ordinator for her invaluable guidance, motivation and help throughout the study. -

Mallory Prelims 27/1/05 1:16 Pm Page I
Mallory Prelims 27/1/05 1:16 pm Page i Illustrated Manual of Pediatric Dermatology Mallory Prelims 27/1/05 1:16 pm Page ii Mallory Prelims 27/1/05 1:16 pm Page iii Illustrated Manual of Pediatric Dermatology Diagnosis and Management Susan Bayliss Mallory MD Professor of Internal Medicine/Division of Dermatology and Department of Pediatrics Washington University School of Medicine Director, Pediatric Dermatology St. Louis Children’s Hospital St. Louis, Missouri, USA Alanna Bree MD St. Louis University Director, Pediatric Dermatology Cardinal Glennon Children’s Hospital St. Louis, Missouri, USA Peggy Chern MD Department of Internal Medicine/Division of Dermatology and Department of Pediatrics Washington University School of Medicine St. Louis, Missouri, USA Mallory Prelims 27/1/05 1:16 pm Page iv © 2005 Taylor & Francis, an imprint of the Taylor & Francis Group First published in the United Kingdom in 2005 by Taylor & Francis, an imprint of the Taylor & Francis Group, 2 Park Square, Milton Park Abingdon, Oxon OX14 4RN, UK Tel: +44 (0) 20 7017 6000 Fax: +44 (0) 20 7017 6699 Website: www.tandf.co.uk All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP. Although every effort has been made to ensure that all owners of copyright material have been acknowledged in this publication, we would be glad to acknowledge in subsequent reprints or editions any omissions brought to our attention. -

Otolaryngology-Head and Neck Surgery Clinical
OTOLARYNGOLOGY HEAD &NECK SURGERY CLINICAL REFERENCE GUIDE Fifth Edition OTOLARYNGOLOGY HEAD &NECK SURGERY CLINICAL REFERENCE GUIDE Fifth Edition Raza Pasha, MD Justin S. Golub, MD, MS 5521 Ruffin Road San Diego, CA 92123 e-mail: [email protected] Website: www.pluralpublishing.com Copyright © 2018 by Plural Publishing, Inc. Typeset in 9/11 Adobe Garamond Pro by Flanagan’s Publishing Services, Inc. Printed in the United States of America by McNaughton & Gunn All rights, including that of translation, reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, recording, or otherwise, including photocopying, recording, taping, Web distribution, or information storage and retrieval systems without the prior written consent of the publisher. For permission to use material from this text, contact us by Telephone: (866) 758-7251 Fax: (888) 758-7255 e-mail: [email protected] Every attempt has been made to contact the copyright holders for material originally printed in another source. If any have been inadvertently overlooked, the publishers will gladly make the necessary arrangements at the first opportunity. NOTICE TO THE READER Care has been taken to confirm the accuracy of the indications, procedures, drug dosages, and diagnosis and remediation protocols presented in this book and to ensure that they conform to the practices of the general medical and health services communities. However, the authors, editors, and publisher are not responsible for errors or omissions or for any consequences from application of the information in this book and make no warranty, expressed or implied, with respect to the currency, completeness, or accuracy of the contents of the publication. -

View the 2019 Index
Index A Abnormal uterine bleeding (AUB), heart failure, 311 Achondroplasia, 454 A-a gradient 618, 619 hypertension, 319 chromosome disorder, 64 in elderly, 654 adenomyosis, 634 naming convention for, 253 endochondral ossification in, 450 with hypoxemia, 654, 655 anemia with, 410 preload/afterload effects, 282 inheritance, 60 restrictive lung disease, 661 Asherman syndrome, 634 teratogenicity, 600 AChR (acetylcholine receptor), 229 Abacavir, 201, 203 leiomyoma (fibroid), 634 Acetaldehyde, 72 Acid-base physiology, 580 Abciximab, 122 polyps (endometrial), 634 Acetaldehyde dehydrogenase, 70, 72 Acidemia, 580 Glycoprotein IIb/IIIa inhibitors, thecoma, 632 Acetaminophen, 474 diuretic effect on, 595 429 ABO blood classification, 397 vs. aspirin for pediatric patients, 474 Acid-fast oocysts, 177 thrombogenesis and, 403 newborn hemolysis, 397 free radical injury and, 210 Acid-fast organisms, 125, 140, 155 Abdominal aorta, 357 Abruptio placentae, 626 hepatic necrosis from, 249 Acidic amino acids, 81 atherosclerosis in, 305, 687 cocaine use, 600 N-acetylcysteine for overdose, 671 Acid maltase, 86 bifurcation of, 649 preeclampsia, 629 for osteoarthritis, 458 Acidosis, 578, 580 Abdominal aortic aneurysm, 306 Abscess, 470 toxicity effects, 474 contractility in, 282 Abdominal colic acute inflammation and, 215 toxicity treatment for, 247 hyperkalemia with, 578 lead poisoning, 411 lung, 670 Acetazolamide, 252, 539, 594 Acid phosphatase in neutrophils, 398 Abdominal pain Absence seizures idiopathic intracranial Acid reflux bacterial peritonitis, 384 characteristics -

Osteopathic Manipulative Medicine for Inflammatory Skin Diseases
Volume 31 JAOCDJournal Of The American Osteopathic College Of Dermatology Focus on Osteopathy: Osteopathic Manipulative Medicine for Inflammatory Skin Diseases Also in this issue: Lichen Planopilaris: A Therapeutic Management Review Clinical Manifestations of Livedoid Vasculopathy “8 to Z” Yin and Yang: A Novel Double Rotation Flap last modified on December 19, 2014 10:29 AM JOURNAL OF THE AMERICAN OSTEOPATHIC COLLEGE OF DERMATOLOGY Page 1 JOURNAL OF THE AMERICAN OSTEOPATHIC COLLEGE OF DERMATOLOGY 2014-2015 AOCD OFFICERS PRESIDENT Rick Lin, DO, FAOCD PRESIDENT-ELECT Alpesh Desai, DO, FAOCD FIRST VICE-PRESIDENT Karthik Krishnamurthy, DO, FAOCD SECOND VICE-PRESIDENT Daniel Ladd, DO, FAOCD THIRD VICE-PRESIDENT John P. Minni, DO, FAOC Editor-in-Chief SECRETARY-TREASURER Karthik Krishnamurthy, DO Jere J. Mammino, DO, FAOCD TRUSTEES Danica Alexander, DO, FAOCD (2012-2015) Reagan Anderson, DO, FAOCD (2012-2015) Michael Whitworth, DO, FAOCD (2013-2016) Tracy Favreau, DO, FAOCD (2013-2016) Sponsors: David Cleaver, DO, FAOCD (2014-2017) Amy Spizuoco, DO, FAOCD (2014-2017) Bayer Immediate Past-President AuroraDx Suzanne Sirota Rozenberg, DO, FAOCD EEC Representatives Ranbaxy James Bernard, DO, FAOCD Michael Scott, DO, FAOCD Valeant Finance Committee Representative Steven K. Grekin, DO, FAOCD AOBD Representative Stephen Purcell, DO, FAOCD Executive Director Marsha A. Wise, BS AOCD • 2902 N. Baltimore St. • Kirksville, MO 63501 800-449-2623 • FAX: 660-627-2623 • www.aocd.org COPYRIGHT AND PERMISSION: Written permission must be obtained from the Journal of the American Osteopathic College of Dermatology for copying or reprinting text of more than half a page, tables or figures. Permissions are normally granted contingent upon similar permission from the author(s), inclusion of acknowledgment of the original source, and a payment of $15 per page, table or figure of reproduced material. -

The Usual Suspects: Rosacea, Acne, Lichen Planus, Psoriasis, Contact Dermatitis CYNTHIA GRIFFITH MPAS, PA-C Rosacea
The Usual Suspects: Rosacea, Acne, Lichen Planus, Psoriasis, Contact Dermatitis CYNTHIA GRIFFITH MPAS, PA-C Rosacea Erythematelangectatic Papulopustular Phymatous Rosacea . Rosacea is a chronic inflammatory condition of the face, which may present with easy flushing, erythema, telangiectasias, papules and pustules, and/or phymatous changes . Can have Ocular involvement: Blepharitis, FB sensation, burning, stinging, dryness, blurred vision, styes, corneal ulceration (refer to Opthomology) . No comedones, unrelated to hormones. Triggers: sun, heat, emotion chemical irritation, alcohol, strong drinks, spices Rosacea . Topical treatments: Metronidazole topical gel or cream, Sodium Sulfacetamide with %5 sulfur, Azelaic acid . Oral treatments: Tetracyclines, macrolides . Lasers: Pulse dye laser (Vbeam laser), Intense pulse light laser . All patients with rosacea should use sunscreen . Steroids can worsen or induce rosacea Acne Vulgaris Acne Vulgaris Primary lesion: Comedone open and closed comedones, papules, pustules, nodules, and cysts . Include the following when describing . morphology . Comedonal vs Inflammatory (either papular/pustular or nodulocystic) or mixed) . severity (Mild, Moderate, Severe) . presence of scarring . Pathogenesis of acne vulgaris is related to the presence of androgens, excess sebum production, the activity of P. acnes, and follicular hyperkeratinization JAMES, WILLIAM D., DIRK M. ELSTON, TIMOTHY G. BERGER, AND GEORGE CLINTON ANDREWS. “ACNE." ANDREWS' DISEASES OF THE SKIN: CLINICAL DERMATOLOGY. 11TH ED. [LONDON]: -

Infectious Diseases in Critical Care Medicine
Infectious Diseases in Critical Care Medicine Cunha_978-1420092400_TP.indd 1 10/5/09 4:21:18 PM INFECTIOUS DISEASE AND THERAPY Series Editor Burke A. Cunha Winthrop-University Hospital Mineola, New York and State University of New York School of Medicine Stony Brook, New York 1. Parasitic Infections in the Compromised Host, edited by Peter D. Walter and Robert M. Genta 2. Nucleic Acid and Monoclonal Antibody Probes: Applications in Diagnostic Method- ology, edited by Bala Swaminathan and Gyan Prakash 3. Opportunistic Infections in Patients with the Acquired Immunodeficiency Syndrome, edited by Gifford Leoung and John Mills 4. Acyclovir Therapy for Herpesvirus Infections, edited by David A. Baker 5. The New Generation of Quinolones, edited by Clifford Siporin, Carl L. Heifetz, and John M. Domagala 6. Methicillin-Resistant Staphylococcus aureus: Clinical Management and Laboratory Aspects, edited by Mary T. Cafferkey 7. Hepatitis B Vaccines in Clinical Practice, edited by Ronald W. Ellis 8. The New Macrolides, Azalides, and Streptogramins: Pharmacology and Clinical Applications, edited by Harold C. Neu, Lowell S. Young, and Stephen H. Zinner 9. Antimicrobial Therapy in the Elderly Patient, edited by Thomas T. Yoshikawa and Dean C. Norman 10. Viral Infections of the Gastrointestinal Tract: Second Edition, Revised and Expanded, edited by Albert Z. Kapikian 11. Development and Clinical Uses of Haemophilus b Conjugate Vaccines, edited by Ronald W. Ellis and Dan M. Cranoff 12. Pseudomonas aeruginosa Infections and Treatment, edited by Aldona L. Battch and Raymond P. Smith 13. Herpesvirus Infections, edited by Ronald Glaser and James F. Jones 14. Chronic Fatigue Syndrome, edited by Stephen E. -

SNODENT (Systemized Nomenclature of Dentistry)
ANSI/ADA Standard No. 2000.2 Approved by ANSI: December 3, 2018 American National Standard/ American Dental Association Standard No. 2000.2 (2018 Revision) SNODENT (Systemized Nomenclature of Dentistry) 2018 Copyright © 2018 American Dental Association. All rights reserved. Any form of reproduction is strictly prohibited without prior written permission. ADA Standard No. 2000.2 - 2018 AMERICAN NATIONAL STANDARD/AMERICAN DENTAL ASSOCIATION STANDARD NO. 2000.2 FOR SNODENT (SYSTEMIZED NOMENCLATURE OF DENTISTRY) FOREWORD (This Foreword does not form a part of ANSI/ADA Standard No. 2000.2 for SNODENT (Systemized Nomenclature of Dentistry). The ADA SNODENT Canvass Committee has approved ANSI/ADA Standard No. 2000.2 for SNODENT (Systemized Nomenclature of Dentistry). The Committee has representation from all interests in the United States in the development of a standardized clinical terminology for dentistry. The Committee has adopted the standard, showing professional recognition of its usefulness in dentistry, and has forwarded it to the American National Standards Institute with a recommendation that it be approved as an American National Standard. The American National Standards Institute granted approval of ADA Standard No. 2000.2 as an American National Standard on December 3, 2018. A standard electronic health record (EHR) and interoperable national health information infrastructure require the use of uniform health information standards, including a common clinical language. Data must be collected and maintained in a standardized format, using uniform definitions, in order to link data within an EHR system or share health information among systems. The lack of standards has been a key barrier to electronic connectivity in healthcare. Together, standard clinical terminologies and classifications represent a common medical language, allowing clinical data to be effectively utilized and shared among EHR systems.