Biokinetics of Cadmium and Zinc Accumulation and Depuration at Different Stages in the Life Cycle of the Cuttlefish Sepia Offici
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
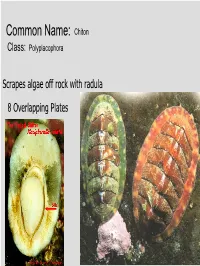
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Octopus Insularis</Italic> As a New Marine Model for Evolutionary
© 2019. Published by The Company of Biologists Ltd | Biology Open (2019) 8, bio046086. doi:10.1242/bio.046086 RESEARCH ARTICLE Octopus insularis as a new marine model for evolutionary developmental biology Ernesto Maldonado1,*, Emma Rangel-Huerta1,2, Roberto González-Gómez3,4, Gabriel Fajardo-Alvarado3,4 and Piedad S. Morillo-Velarde4,5,* ABSTRACT of aquatic animal eggs and embryos guarantees the observation of Octopuses are intriguing organisms that, together with squids and every developmental stage using microscopy and allows detailed cuttlefishes, form the extant coleoid cephalopods. This group includes experimental analysis from the first cell division through to the many species that can potentially be used as models in the fields of formation of embryonic germ layers and organogenesis (Boletzky biomedicine, developmental biology, evolution, neuroscience and et al., 2006). Finally, small embryos allow reasonable sample sizes even for robotics research. The purpose of this work is to first to be tested together using multi-well plates to provide multiple present a simple method for maintaining Octopus insularis embryos experimental replicates at the same time, making them cost- under a laboratory setup. Second, we show that these embryos are effective animal models (Hill et al., 2005). suitable for detailed analyses of specific traits that appear during Coleoid cephalopods (octopus, squid and cuttlefish) exhibit the developmental stages, including the eyes, hearts, arms, suckers, largest nervous systems found among invertebrates (Young, 1971) chromatophores and Kölliker’s organs. Similar complex traits between and a sophisticated visual system controlling body color changes for cephalopods and vertebrates such as the visual, cardiovascular, communication, camouflage and mimicry (Hanlon et al., 2011; neural and pigmentation systems are generally considered to be a Robin et al., 2014). -

1. in Tro Duc Tion
Cephalopods of the World 1 1. INTRO DUC TION Patrizia Jereb, Clyde F.E. Roper and Michael Vecchione he increasing exploitation of finfish resources, and the commercial status. For example, this work should be useful Tdepletion of a number of major fish stocks that formerly for the ever-expanding search for development and supported industrial-scale fisheries, forces continued utilization of ‘natural products’, pharmaceuticals, etc. attention to the once-called ‘unconventional marine resources’, which include numerous species of cephalopods. The catalogue is based primarily on information available in Cephalopod catches have increased steadily in the last 40 published literature. However, yet-to-be-published reports years, from about 1 million metric tonnes in 1970 to more than and working documents also have been used when 4 million metric tonnes in 2007 (FAO, 2009). This increase appropriate, especially from geographical areas where a confirms a potential development of the fishery predicted by large body of published information and data are lacking. G.L. Voss in 1973, in his first general review of the world’s We are particularly grateful to colleagues worldwide who cephalopod resources prepared for FAO. The rapid have supplied us with fisheries information, as well as expansion of cephalopod fisheries in the decade or so bibliographies of local cephalopod literature. following the publication of Voss’s review, meant that a more comprehensive and updated compilation was required, The fishery data reported herein are taken from the FAO particularly for cephalopod fishery biologists, zoologists and official database, now available on the Worldwide web: students. The FAO Species Catalogue, ‘Cephalopods of the FISHSTAT Plus 2009. -

Lab 5: Phylum Mollusca
Biology 18 Spring, 2008 Lab 5: Phylum Mollusca Objectives: Understand the taxonomic relationships and major features of mollusks Learn the external and internal anatomy of the clam and squid Understand the major advantages and limitations of the exoskeletons of mollusks in relation to the hydrostatic skeletons of worms and the endoskeletons of vertebrates, which you will examine later in the semester Textbook Reading: pp. 700-702, 1016, 1020 & 1021 (Figure 47.22), 943-944, 978-979, 1046 Introduction The phylum Mollusca consists of over 100,000 marine, freshwater, and terrestrial species. Most are familiar to you as food sources: oysters, clams, scallops, and yes, snails, squid and octopods. Some also serve as intermediate hosts for parasitic trematodes, and others (e.g., snails) can be major agricultural pests. Mollusks have many features in common with annelids and arthropods, such as bilateral symmetry, triploblasty, ventral nerve cords, and a coelom. Unlike annelids, mollusks (with one major exception) do not possess a closed circulatory system, but rather have an open circulatory system consisting of a heart and a few vessels that pump blood into coelomic cavities and sinuses (collectively termed the hemocoel). Other distinguishing features of mollusks are: z A large, muscular foot variously modified for locomotion, digging, attachment, and prey capture. z A mantle, a highly modified epidermis that covers and protects the soft body. In most species, the mantle also secretes a shell of calcium carbonate. z A visceral mass housing the internal organs. z A mantle cavity, the space between the mantle and viscera. Gills, when present, are suspended within this cavity. -

Squid Dissection
SQUID DISSECTION FOR THE TEACHER Discipline Biological Science Theme Scale and Structure Key Concept The investigation of the structure and function of an open ocean animal like the squid can be used to study adaptations for a completely pelagic existence. Synopsis Students work in pairs to dissect a squid and investigate its structure and how all the parts function together to allow the squid to survive and thrive in its open ocean environment. The squid is then honored as the students participate in a Squid Feast. Science Process Skills observing, communicating, comparing, classifying, relating Social Skill Checking for Understanding Vocabulary benthic, cephalopod, invertebrate, mollusc, pelagic, planktonic MATERIALS For INTO the Activities: Monterey Bay Aquarium Video Collection, "Seasons of the Squid" segment (Available for check-out from the MARE library or for purchase from Monterey Bay Aquarium) Squid Dissection 82 ©2001 The Regents of the University of California Anticipatory Chart #1 and #2 (see charts below) copied on large flip chart paper or on the board: Squid Dissection 83 ©2001 The Regents of the University of California For THROUGH the Activities: For each pair of students and one for yourself: One Squid. Available in grocery stores frozen in 3 lb. boxes for about $1 - $1.50/lb. There are about 32-35 squid per box or enough for two classes. Squid are also available fresh in fish markets. Frozen squid are the number one choice, but if only fresh are available, choose the largest and freshest ones and make sure they haven't been cleaned. Keep them in the freezer until the morning you are going to use them. -

Notes on the Carboniferous Cephalopoda
Downloaded from http://pygs.lyellcollection.org/ by guest on October 1, 2021 257 some two miles inland, at an elevation of about 200 feet above the sea-level; and more recently have had a specimen given to me, said to have been found in Cornwall, but I am somewhat dubious about the accuracy of this locality. I am under the impression that Yermiculite Granite will be found in Yorkshire, especially in the northern division of the West Riding; and I trust our geological friends will carefully examine any granite boulders they may meet with in their excursions. It is only when the Granite is decomposed that the mineral can be properly separated from it; but even in situ, by careful observation, there will be no difficulty in detecting it. That these Granite boulders have travelled a long distance I am fully persuaded, and my belief is they have been transported to our shores in floating ice, very probably from Labrador. NOTES ON CARBONIFEROUS CEPHALOPODA. PART I. RECENT CEPHALOPODA. BY WILLIAM CASH, F.G.S. (PLATE XI.) THE true method of reasoning on natural objects and phenomena, is from that which is well known to that which is unknown; hence it follows, that a sound knowledge of the structure and relations of Fossil Animals can only be acquired by a careful study of the structure and relations of their living analogues. These and similar considerations have resulted in prefacing the proposed " Notes on Carboniferous Cepha• lopoda," with the following remarks on the structure, habits, distribution, and classification of their living repre• sentatives. -

Fish Bulletin 131. the Structure, Development, Food Relations, Reproduction, and Life History of the Squid Loligo Opalescens Berry
UC San Diego Fish Bulletin Title Fish Bulletin 131. The Structure, Development, Food Relations, Reproduction, and Life History of the Squid Loligo opalescens Berry Permalink https://escholarship.org/uc/item/4q30b714 Author Fields, W Gordon Publication Date 1964-05-01 eScholarship.org Powered by the California Digital Library University of California STATE OF CALIFORNIA THE RESOURCES AGENCY DEPARTMENT OF FISH AND GAME FISH BULLETIN 131 The Structure, Development, Food Relations, Reproduction, and Life History of the Squid Loligo opalescens Berry By W. GORDON FIELDS 1965 1 2 3 4 ACKNOWLEDGMENTS This study ("a dissertation submitted to the Department of Biological Sciences and the Committee on the Graduate Division of Stanford University in partial fulfillment of the requirements for the degree of Doctor of Philosophy") was undertaken at the suggestion, and under the direction, of Tage Skogsberg, Hopkins Marine Station, to whom I am greatly indebted for inspiration and guidance until his untimely death. Thereafter, Rolf L. Bolin was my advisor, and I wish to express my appreciation to him for the help he gave me. In the final part of this investigation and in bringing its results together, Donald P. Abbott, through his enthusiasm, his wide scientific knowledge, his ability and his wisdom, has given encouragement and immeasurable help in many ways: to him I express my deepest appre- ciation. Lawrence R. Blinks, Director of the Hopkins Marine Station, throughout my work there, arranged suitable laboratory space and conditions for my use and, deftly and to my advantage, resolved all administrative matters re- ferred to him; I am very grateful to him. -

Uptake, Transfer and Distribution of Silver and Cobalt in Tissues of the Common Cuttlefish Sepia Officinalis at Different Stages of Its Life Cycle
MARINE ECOLOGY PROGRESS SERIES Vol. 269: 185–195, 2004 Published March 25 Mar Ecol Prog Ser Uptake, transfer and distribution of silver and cobalt in tissues of the common cuttlefish Sepia officinalis at different stages of its life cycle P. Bustamante1,*, J.-L. Teyssié2, B. Danis3, S. W. Fowler 2, P. Miramand1, O. Cotret 2, M. Warnau2 1Laboratoire de Biologie et Environnement Marins, FRE 2727 du CNRS, Université de La Rochelle, 22, Avenue Michel Crépeau, 17042 La Rochelle, France 2Marine Environment Laboratory, International Atomic Energy Agency, 4 Quai Antoine Ier, 98000, Monaco 3Laboratoire de Biologie Marine, CP 160-15, Université Libre de Bruxelles, 50 Avenue F.D. Roosevelt, 1050 Bruxelles, Belgium ABSTRACT: Three pathways of exposure (sediment, seawater and food) were examined to deter- mine transfer of 110mAg and 57Co in juvenile cuttlefish Sepia officinalis. Additional experiments were conducted on adult cuttlefish and their eggs/embryos in order to assess bioaccumulation patterns at different stages of the organism’s life cycle. Eggs, juveniles and adults readily accumulated both Ag and Co from seawater. In eggs, both metals were predominantly adsorbed onto the capsule mem- brane (≥60% for Ag and ≥99% for Co), indicating that the latter may act as an effective shield to limit exposure of embryos to soluble metals. Adult cuttlefish incorporated waterborne radiotracers mainly in their muscular tissues (≥60% of the whole-body burden); subsequent metal retention was greater for Co (biological half-life, Tb! = 34 d) than for Ag (Tb! = 7 d). Turnover of Co ingested with food was much more rapid in juveniles (Tb! = 5 d) than in adults (Tb! = 990 d), suggesting that the functional maturation of the digestive gland was not complete in the juveniles. -

A Studyguide by Andrew Fildes
the brainy bunch A STUDYGUIDE B Y A NDREW FILDES www.metromagazine.com.au www.theeducationshop.com.au the brainy bunch Introduction squid, their close relatives and they are members of the cephalopod (head-footer) group of molluscs, Imagine a creature so alien that it has which also includes octopuses. The octopus is noted for its intelligence three hearts, no bones, blue blood, ten and generally believed to be the arms sticking out of its head, can fly smartest invertebrate, but are these little beasts just as bright? by jet propulsion and can make itself ‘When you come across your first cuttlefish, they’re the most invisible; or even change its own shape amazing creatures … they’re this sort of small, alien space ship and and colour. It sounds too bizarre for you couldn’t get a weirder looking animal underwater.’ (Dr Mark a Star Wars episode and yet it exists Norman, Museum Victoria) for real, right now and not far from This remarkable documentary explores the ecology, psychology you. In fact, the seas teem with them and biology of this amazing group of creatures. And there is plenty to – cuttlefish. study because it is hard to imagine another genus that has developed ou’ve seen the evidence such an extraordinary range of of their short lives on the physical characteristics and social beach, the light, white, behaviours. While it is of interest Y chalky shells (plates) that to middle and senior level biology budgies love to chew and yet few students, it is also of interest to of us have seen them in the wild. -

Phylum Mollusca
Animal Diversity: (Non-Chordates) Phylum : Mollusca Ranjana Saxena Associate Professor, Department of Zoology, Dyal Singh College, University of Delhi Delhi e-mail: [email protected] 24th September 2007 CONTENT 1. GENERAL CHARACTERISTICS 2. PILA GLOBOSA a) Habit and Habitat b) Morphology c) Coelom d) Locomotion e) Digestive System f) Respiratory system g) Circulatory System h) Excretory System i) Nervous System j) Sense organs k) Reproductive System 3. SEPIA a) Habit and Habitat b) Morphology c) Shell d) Coelom e) Locomotion f) Digestive System g) Respiratory System h) Circulatory System i) Excretory System j) Nervous System k) Sense Organs l) Reproductive System 4. ANCESTRAL MOLLUSK 5. SHELL IN MOLLUSCA 6. FOOT AND ITS MODIFICATION 2 7. GILLS AND ITS MODIFICATION 8. MANTLE 9. TORSION IN MOLLUSCA 10. PEARL FORMATION 11. CLASSIFICATION 12. BIBLIOGRAPHY 13. SUGGESTED READING 3 PHYLUM MOLLUSCA The word Mollusca is derived from the latin word mollis which means soft bodied. GENERAL CHARACTERISTICS • It is the second largest phylum of invertebrates consisting of more than 80,000 living species and about 35,000 fossil species. • The adults are triploblastic, bilaterally symmetrical animals with a soft unsegmented body. However, the bilateral symmetry may be lost in some adult mollusc. • Majority of them are enclosed in a calcareous shell. The shell may be external or in a few molluscs it may be internal, reduced or absent. • They have a well marked cephalisation. • The body is divisible into head, mantle, foot and visceral mass. • The visceral mass is enclosed in a thick muscular fold of the body wall called mantle which secretes the shell. -

Development of the Ommastrephid Squid Todarodes Pacificus, from Fertilized Egg to the Rhynchoteuthion Paralarva
Title Development of the ommastrephid squid Todarodes pacificus, from fertilized egg to the rhynchoteuthion paralarva Author(s) Watanabe, Kumi; Sakurai, Yasunori; Segawa, Susumu; Okutani, Takashi Citation American Malacological Bulletin, 13(1/2), 73-88 Issue Date 1996 Doc URL http://hdl.handle.net/2115/35243 Type article File Information sakurai-23.pdf Instructions for use Hokkaido University Collection of Scholarly and Academic Papers : HUSCAP Development of the ommastrephid squid Todarodes pacific us , from fertilized egg to rhynchoteuthion paralarva Kunli Watanabel , Yasunori Sakurai2, Susumu Segawal , and Takashi Okutani3 lLaboratory of Invertebrate Zoology, Tokyo University of Fisheries, Konan, Minato-ku, Tokyo 108, Japan 2Faculty of Fisheries, Hokkaido University, Minatocho, Hakodate, Hokkaido 041, Japan 3College of Bioresource Sciences, Nihon University Fujisawa City, Kanagawa 252, Japan Abstract: The present study establishes for the first time an atlas for the normal development of Todarodes pacificus Steenstrup, 1880, from fertilized egg to rhynchoteuthion paralarva. In the course of the study, observations on embryogenesis and histological differentiation in T. pacificus were made for consideration of the developmental mode of the Oegopsida, which is a specialized group with a reduced external yolk sac. It appears that differentiation of the respiratory and digestive organs is relatively delayed in the Oegopsida, with reduction of the yolk sac as well as the egg size. These characters could be related to a reproductive strategy -

Cephalopod Guidelines
Reference Resources Caveats from AAALAC’s Council on Accreditation regarding this resource: Guidelines for the Care and Welfare of Cephalopods in Research– A consensus based on an initiative by CephRes, FELASA and the Boyd Group *This reference was adopted by the Council on Accreditation with the following clarification and exceptions: The AAALAC International Council on Accreditation has adopted the “Guidelines for the Care and Welfare of Cephalopods in Research- A consensus based on an initiative by CephRes, FELASA and the Boyd Group” as a Reference Resource with the following two clarifications and one exception: Clarification: The acceptance of these guidelines as a Reference Resource by AAALAC International pertains only to the technical information provided, and not the regulatory stipulations or legal implications (e.g., European Directive 2010/63/EU) presented in this article. AAALAC International considers the information regarding the humane care of cephalopods, including capture, transport, housing, handling, disease detection/ prevention/treatment, survival surgery, husbandry and euthanasia of these sentient and highly intelligent invertebrate marine animals to be appropriate to apply during site visits. Although there are no current regulations or guidelines requiring oversight of the use of invertebrate species in research, teaching or testing in many countries, adhering to the principles of the 3Rs, justifying their use for research, commitment of appropriate resources and institutional oversight (IACUC or equivalent oversight body) is recommended for research activities involving these species. Clarification: Page 13 (4.2, Monitoring water quality) suggests that seawater parameters should be monitored and recorded at least daily, and that recorded information concerning the parameters that are monitored should be stored for at least 5 years.