Cephalopod Tissue Regeneration: Consolidating Over a Century of Knowledge
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CEPHALOPODS 688 Cephalopods
click for previous page CEPHALOPODS 688 Cephalopods Introduction and GeneralINTRODUCTION Remarks AND GENERAL REMARKS by M.C. Dunning, M.D. Norman, and A.L. Reid iving cephalopods include nautiluses, bobtail and bottle squids, pygmy cuttlefishes, cuttlefishes, Lsquids, and octopuses. While they may not be as diverse a group as other molluscs or as the bony fishes in terms of number of species (about 600 cephalopod species described worldwide), they are very abundant and some reach large sizes. Hence they are of considerable ecological and commercial fisheries importance globally and in the Western Central Pacific. Remarks on MajorREMARKS Groups of CommercialON MAJOR Importance GROUPS OF COMMERCIAL IMPORTANCE Nautiluses (Family Nautilidae) Nautiluses are the only living cephalopods with an external shell throughout their life cycle. This shell is divided into chambers by a large number of septae and provides buoyancy to the animal. The animal is housed in the newest chamber. A muscular hood on the dorsal side helps close the aperture when the animal is withdrawn into the shell. Nautiluses have primitive eyes filled with seawater and without lenses. They have arms that are whip-like tentacles arranged in a double crown surrounding the mouth. Although they have no suckers on these arms, mucus associated with them is adherent. Nautiluses are restricted to deeper continental shelf and slope waters of the Indo-West Pacific and are caught by artisanal fishers using baited traps set on the bottom. The flesh is used for food and the shell for the souvenir trade. Specimens are also caught for live export for use in home aquaria and for research purposes. -
Common Name: Chiton Class: Polyplacophora
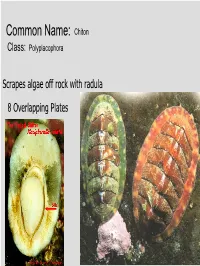
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Nautiloid Shell Morphology
MEMOIR 13 Nautiloid Shell Morphology By ROUSSEAU H. FLOWER STATEBUREAUOFMINESANDMINERALRESOURCES NEWMEXICOINSTITUTEOFMININGANDTECHNOLOGY CAMPUSSTATION SOCORRO, NEWMEXICO MEMOIR 13 Nautiloid Shell Morphology By ROUSSEAU H. FLOIVER 1964 STATEBUREAUOFMINESANDMINERALRESOURCES NEWMEXICOINSTITUTEOFMININGANDTECHNOLOGY CAMPUSSTATION SOCORRO, NEWMEXICO NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Alvin J. Thompson, Director THE REGENTS MEMBERS EXOFFICIO THEHONORABLEJACKM.CAMPBELL ................................ Governor of New Mexico LEONARDDELAY() ................................................... Superintendent of Public Instruction APPOINTEDMEMBERS WILLIAM G. ABBOTT ................................ ................................ ............................... Hobbs EUGENE L. COULSON, M.D ................................................................. Socorro THOMASM.CRAMER ................................ ................................ ................... Carlsbad EVA M. LARRAZOLO (Mrs. Paul F.) ................................................. Albuquerque RICHARDM.ZIMMERLY ................................ ................................ ....... Socorro Published February 1 o, 1964 For Sale by the New Mexico Bureau of Mines & Mineral Resources Campus Station, Socorro, N. Mex.—Price $2.50 Contents Page ABSTRACT ....................................................................................................................................................... 1 INTRODUCTION -

(Eledone Cirrhosa) in Atlantic Iberian Waters
Manuscript + Figure captions Click here to download Manuscript Eledone cirrhosa diet.docx Click here to view linked References 1 Factors affecting the feeding patterns of the horned octopus ( Eledone 2 cirrhosa ) in Atlantic Iberian waters 3 4 M. Regueira 1,2* , Á.Guerra 1, C.M. Fernández-Jardón 3,Á.F. González 1 5 6 1Instituto de Investigaciones Marinas (IIM-CSIC), Eduardo Cabello 6, 36208 Vigo, Spain. 7 2Departamento de Biologia, Universidade de Aveiro. 3810-193 Aveiro, Portugal. 8 3Facultad de Ciencias Económicas y Empresariales, Departamento de Economía Aplicada, Universidad 9 de Vigo, Campus de Vigo. 36310, Vigo, Spain. 10 11 12 *Corresponding author: [email protected] 13 Tel. (+34) 986 23 19 30 14 Fax. (+34) 986 29 27 62 15 16 17 RUNNING TITLE: Feeding patterns of Eledone cirrhosa 18 KEY WORDS: Cephalopods, Eledone cirrhosa , diet, feeding patterns, Atlantic Iberian waters, 19 Multinomial Logistic Regression. 1 20 Abstract The present study combines morphological and molecular analysis of stomach contents 21 (n=2,355) and Multinomial Logistic Regression (MLR) to understand the diet and feeding patterns of the 22 horned octopus Eledone cirrhosa inhabiting Atlantic Iberian waters. Specimens were collected monthly 23 from commercial bottom trawl fisheries between February 2009 and February 2011 in three fishing 24 grounds (North Galicia, West Galicia and North Portugal), located between 40.6 -43. 6°N and 8.6 -7.36°W. 25 Based on stomach analysis, horned octopuses in the region consumed mainly crustaceans, followed by 26 teleost fish, echinoderms, molluscs and polychaetes. Molecular analysis of 14 stomach contents 27 confirmed the visual identification of pre y items as well as cannibalistic events. -

Cambrian Cephalopods
BULLETIN 40 Cambrian Cephalopods BY ROUSSEAU H. FLOWER 1954 STATE BUREAU OF MINES AND MINERAL RESOURCES NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY CAMPUS STATION SOCORRO, NEW MEXICO NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Eugene Callaghan, Director THE REGENTS MEMBERS Ex OFFICIO The Honorable Edwin L. Mechem ...................... Governor of New Mexico Tom Wiley ......................................... Superintendent of Public Instruction APPOINTED MEMBERS Robert W. Botts ...................................................................... Albuquerque Holm 0. Bursum, Jr. ....................................................................... Socorro Thomas M. Cramer ........................................................................ Carlsbad Frank C. DiLuzio ..................................................................... Los Alamos A. A. Kemnitz ................................................................................... Hobbs Contents Page ABSTRACT ...................................................................................................... 1 FOREWORD ................................................................................................... 2 ACKNOWLEDGMENTS ............................................................................. 3 PREVIOUS REPORTS OF CAMBRIAN CEPHALOPODS ................ 4 ADEQUATELY KNOWN CAMBRIAN CEPHALOPODS, with a revision of the Plectronoceratidae ..........................................................7 -

Siphuncular Structure in the Extant Spirula and in Other Coleoids (Cephalopoda)
GFF ISSN: 1103-5897 (Print) 2000-0863 (Online) Journal homepage: http://www.tandfonline.com/loi/sgff20 Siphuncular Structure in the Extant Spirula and in Other Coleoids (Cephalopoda) Harry Mutvei To cite this article: Harry Mutvei (2016): Siphuncular Structure in the Extant Spirula and in Other Coleoids (Cephalopoda), GFF, DOI: 10.1080/11035897.2016.1227364 To link to this article: http://dx.doi.org/10.1080/11035897.2016.1227364 Published online: 21 Sep 2016. Submit your article to this journal View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=sgff20 Download by: [Dr Harry Mutvei] Date: 21 September 2016, At: 11:07 GFF, 2016 http://dx.doi.org/10.1080/11035897.2016.1227364 Siphuncular Structure in the Extant Spirula and in Other Coleoids (Cephalopoda) Harry Mutvei Department of Palaeobiology, Swedish Museum of Natural History, Box 50007, SE-10405 Stockholm, Sweden ABSTRACT ARTICLE HISTORY The shell wall in Spirula is composed of prismatic layers, whereas the septa consist of lamello-fibrillar nacre. Received 13 May 2016 The septal neck is holochoanitic and consists of two calcareous layers: the outer lamello-fibrillar nacreous Accepted 23 June 2016 layer that continues from the septum, and the inner pillar layer that covers the inner surface of the septal KEYWORDS neck. The pillar layer probably is a structurally modified simple prisma layer that covers the inner surface of Siphuncular structures; the septal neck in Nautilus. The pillars have a complicated crystalline structure and contain high amount of connecting rings; Spirula; chitinous substance. -

Cuttlebone: Characterization and Applications
Cuttlebone: Characterization and Applications By: Safieh Momeni Cuttlebone Cuttlebone signifies a special class of ultra-lightweight, high stiffness and high permeability cellular biomaterials, providing the cuttlefish with an efficient means of maintaining neutral buoyancy at considerable habitation depths. In addition, this rigid cellular material provides the structural backbone of the body and plays a key role in the protection of vital organs. Cuttlebone The cuttlebone has two main components: Dorsal shield Lamellar matrix The dorsal shield is very tough and dense, providing a rigid substrate for protection, structure and the development of the lamellar matrix of cuttlebone. The lamellar matrix of cuttlebone has an extreme porosity (up to 90%), but also manages to withstand very high hydrostatic pressure. Lamellar matrix The lamellar matrix consists primarily of aragonite (a crystallised form of calcium carbonate, CaCO3), enveloped in a layer of organic material composed primary of β-chitin. The organic layer entirely envelopes the inorganic ceramic, and is thought to initiate, organise and inhibit the mineralisation of the inorganic material. From a mechanical perspective, the organic layer is also thought to provide a certain toughening effect to the material Applications Due to the unique physical, chemical and mechanical characteristics of this natural cellular material, a range of novel applications for the material have recently been investigated. Preparation of highly porous hydroxyapatite from cuttlefish bone Hydroxyapatite structures for tissue engineering applications have been produced by hydrothermal (HT) treatment of aragonite in the form of cuttlefish bone at 200 ̊C. Aragonite (CaCO3) monoliths were completely transformed into hydroxyapatite after 48 h of HT treatment. -

Female Description of the Hydrothermal Vent Cephalopod Vulcanoctopus Hydrothermalis A.F
Journal of the Marine Biological Association of the United Kingdom, 2008, 88(2), 375–379. #2008 Marine Biological Association of the United Kingdom doi:10.1017/S0025315408000647 Printed in the United Kingdom Female description of the hydrothermal vent cephalopod Vulcanoctopus hydrothermalis a.f. gonzÆlez1, a. guerra1, s. pascual1 and m. segonzac2 1ECOBIOMAR, Instituto de Investigaciones Marinas (CSIC), Eduardo Cabello 6, 36208 Vigo, Spain, 2IFREMER, Centre de Brest, Laboratoire Environnement Profond, BP 70, 29280-Plouzane´, France During biological sampling of hydrothermal vents on the East Pacific Rise, the manned submersible ‘Nautile’ caught the first female of the endemic cephalopod Vulcanoctopus hydrothermalis. The specimen caught at the vent site Gromit (21833 660S, 114817 980W at 2832 m depth) is described here in detail and an amended diagnosis of the species proposed. The external morphology, measurements and internal structure resemble that of males of this species. One of the most remarkable char- acters is the lack of spermathecae and the absence of apical filaments in the oocytes to provide a site for sperm storage. It is suggested that some species of the genera Benthoctopus and Bathypolypus would be the most suitable octopod ancestor of V. hydrothermalis. Keywords: hydrothermal vent, cephalopods, Vulcanoctopus hydrothermalis, female description Submitted 20 April 2007; accepted 29 November 2007 INTRODUCTION et al., 2006). It inhabits an isolated extreme environment very close to the base of the chimneys and is also observed The study of chemosynthetic ecosystems in the deep sea rep- on the pillow lava at several metres from the active areas. resents a challenging issue due to the difficulty of sampling, This benthic species has characters that represent adaptations which involves the use of modern technologies such as either to the deep-sea or to a hydrothermal vent habitat manned submersibles. -

On the Cephalopod Phosphagen by Ernest Baldwin, B.A
222 ON THE CEPHALOPOD PHOSPHAGEN BY ERNEST BALDWIN, B.A. (From the Biochemical Laboratory, Cambridge, and the Marine Biological Station, Tamaris, Var, France.) (Received 8th November, 1932.) (With Four Text-figures.) INTRODUCTION. THE comparative researches of Eggleton & Eggleton(s) on the distribution of phosphagen made it clear that while creatine phosphate is very widely distributed amongst the vertebrates, it is not present in the invertebrates. Shortly afterwards, a new phosphagenic substance was isolated from crab muscle by Meyerhof & Lohmann(n, 12) and shown to be arginine phosphate, while the later work of Lundsgaard (9) has made it certain that this compound plays in these tissues a part exactly analogous to that played by the creatine compound in vertebrate muscles. Later, Meyerhof (10) examined a number of invertebrates, representative of several phyla, and came to the conclusion that arginine phosphate is present in Holothuria, Pecten and Sipunculus. Cephalopod muscle contained no phosphagen. The case of the cephalopods was further examined by Needham, Needham, Baldwin & Yudkin (13), who not only found that the muscles of Sepia and of Octopus do contain phosphagen, but were also able to investigate its ontogeny in the former (14). There seemed no reason to think that the compound present was any- thing other than the arginine compound (8). Ackermann, Holtz & Kutscherw claim to have isolated the copper nitrate salt of arginine from extracts of the cephalopod Eledone moschata, while Okuda(is) has made a similar claim in the case of Loligo breekert, whereas Iseki (7) has been able to isolate no arginine from extracts of Octopus, finding in its place a compound which he isolated in the form of its picrate, and which he thinks may be a methyl agmatine. -

Cuttlefish (Sepia Sp.) Ink Extract As Antibacterial Activity Against Aeromonas Hydrophila
INTERNATIONAL JOURNAL OF SCIENTIFIC & TECHNOLOGY RESEARCH VOLUME 8, ISSUE 11, NOVEMBER 2019 ISSN 2277-8616 Cuttlefish (Sepia Sp.) Ink Extract As Antibacterial Activity Against Aeromonas Hydrophila Faizal Zakaria, Mohamad Fadjar, Uun Yanuhar Abstract: Aeromonas hydrophila is a gram negative opportunist bacterium associated with aquatic animal disease. Cephalopod ink has shown potential antiretroviral activity. The ink extracts of cuttlefish showed antibacterial effect. This study aims to investigate the antibacterial activity of the methanolic extract of the ink of cuttlefish (Sepia sp.) against Aeromonas hydrophilla. The shadedried ink sample from approximately 30g ink sacs obtained from 15 animals were immersed separately in methanol (1:3 w/v) solvents for overnight. Dried extract was used for the experiments. Isolate of Aeromonas hydrophila was originated from Jepara Brackishwater Aquaculture Center. The average yield percentage of cuttlefish tintan extract obtained was 4.86%. The results of the MIC test in table 5. show that the highest average absorbance value was obtained at a concentration of 50 ppm which was equal to 1,716 nm and the lowest absorbance was obtained at a treatment dose of 300 ppm at 0.841 nm while the Mc Farland tube was 0.933 nm. The results of antibacterial test on table 2 showed antibacterial activity of cuttlefish ink extract at concentration negative control showed diameter zone of 5 ± 1.2 mm, at positive control showed diameter zone of 31 ± 1.2 mm, at 250 ppm result 19 ± 0.9 mm, at 300 ppm result 22 ± 1.4 mm, at 350 ppm result 31 ± 1.2 mm. Index Terms: Antibacterial; Cuttlefish Ink; Extract;Sepia sp.;Aeromonas hydrophila —————————— —————————— 1. -

Life History, Mating Behavior, and Multiple Paternity in Octopus
LIFE HISTORY, MATING BEHAVIOR, AND MULTIPLE PATERNITY IN OCTOPUS OLIVERI (BERRY, 1914) (CEPHALOPODA: OCTOPODIDAE) A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAI´I AT MĀNOA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN ZOOLOGY DECEMBER 2014 By Heather Anne Ylitalo-Ward Dissertation Committee: Les Watling, Chairperson Rob Toonen James Wood Tom Oliver Jeff Drazen Chuck Birkeland Keywords: Cephalopod, Octopus, Sexual Selection, Multiple Paternity, Mating DEDICATION To my family, I would not have been able to do this without your unending support and love. Thank you for always believing in me. ii ACKNOWLEDGMENTS I would like to thank all of the people who helped me collect the specimens for this study, braving the rocks and the waves in the middle of the night: Leigh Ann Boswell, Shannon Evers, and Steffiny Nelson, you were the hard core tako hunters. I am eternally grateful that you sacrificed your evenings to the octopus gods. Also, thank you to David Harrington (best bucket boy), Bert Tanigutchi, Melanie Hutchinson, Christine Ambrosino, Mark Royer, Chelsea Szydlowski, Ily Iglesias, Katherine Livins, James Wood, Seth Ylitalo-Ward, Jessica Watts, and Steven Zubler. This dissertation would not have happened without the support of my wonderful advisor, Dr. Les Watling. Even though I know he wanted me to study a different kind of “octo” (octocoral), I am so thankful he let me follow my foolish passion for cephalopod sexual selection. Also, he provided me with the opportunity to ride in a submersible, which was one of the most magical moments of my graduate career. -

Octopus Insularis</Italic> As a New Marine Model for Evolutionary
© 2019. Published by The Company of Biologists Ltd | Biology Open (2019) 8, bio046086. doi:10.1242/bio.046086 RESEARCH ARTICLE Octopus insularis as a new marine model for evolutionary developmental biology Ernesto Maldonado1,*, Emma Rangel-Huerta1,2, Roberto González-Gómez3,4, Gabriel Fajardo-Alvarado3,4 and Piedad S. Morillo-Velarde4,5,* ABSTRACT of aquatic animal eggs and embryos guarantees the observation of Octopuses are intriguing organisms that, together with squids and every developmental stage using microscopy and allows detailed cuttlefishes, form the extant coleoid cephalopods. This group includes experimental analysis from the first cell division through to the many species that can potentially be used as models in the fields of formation of embryonic germ layers and organogenesis (Boletzky biomedicine, developmental biology, evolution, neuroscience and et al., 2006). Finally, small embryos allow reasonable sample sizes even for robotics research. The purpose of this work is to first to be tested together using multi-well plates to provide multiple present a simple method for maintaining Octopus insularis embryos experimental replicates at the same time, making them cost- under a laboratory setup. Second, we show that these embryos are effective animal models (Hill et al., 2005). suitable for detailed analyses of specific traits that appear during Coleoid cephalopods (octopus, squid and cuttlefish) exhibit the developmental stages, including the eyes, hearts, arms, suckers, largest nervous systems found among invertebrates (Young, 1971) chromatophores and Kölliker’s organs. Similar complex traits between and a sophisticated visual system controlling body color changes for cephalopods and vertebrates such as the visual, cardiovascular, communication, camouflage and mimicry (Hanlon et al., 2011; neural and pigmentation systems are generally considered to be a Robin et al., 2014).