The Innervation and Physiology of the Cardiac Tissue in Squid
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
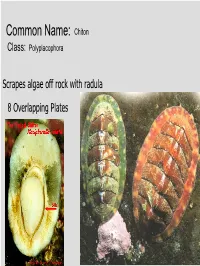
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Nautiloid Shell Morphology
MEMOIR 13 Nautiloid Shell Morphology By ROUSSEAU H. FLOWER STATEBUREAUOFMINESANDMINERALRESOURCES NEWMEXICOINSTITUTEOFMININGANDTECHNOLOGY CAMPUSSTATION SOCORRO, NEWMEXICO MEMOIR 13 Nautiloid Shell Morphology By ROUSSEAU H. FLOIVER 1964 STATEBUREAUOFMINESANDMINERALRESOURCES NEWMEXICOINSTITUTEOFMININGANDTECHNOLOGY CAMPUSSTATION SOCORRO, NEWMEXICO NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Alvin J. Thompson, Director THE REGENTS MEMBERS EXOFFICIO THEHONORABLEJACKM.CAMPBELL ................................ Governor of New Mexico LEONARDDELAY() ................................................... Superintendent of Public Instruction APPOINTEDMEMBERS WILLIAM G. ABBOTT ................................ ................................ ............................... Hobbs EUGENE L. COULSON, M.D ................................................................. Socorro THOMASM.CRAMER ................................ ................................ ................... Carlsbad EVA M. LARRAZOLO (Mrs. Paul F.) ................................................. Albuquerque RICHARDM.ZIMMERLY ................................ ................................ ....... Socorro Published February 1 o, 1964 For Sale by the New Mexico Bureau of Mines & Mineral Resources Campus Station, Socorro, N. Mex.—Price $2.50 Contents Page ABSTRACT ....................................................................................................................................................... 1 INTRODUCTION -

Cambrian Cephalopods
BULLETIN 40 Cambrian Cephalopods BY ROUSSEAU H. FLOWER 1954 STATE BUREAU OF MINES AND MINERAL RESOURCES NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY CAMPUS STATION SOCORRO, NEW MEXICO NEW MEXICO INSTITUTE OF MINING & TECHNOLOGY E. J. Workman, President STATE BUREAU OF MINES AND MINERAL RESOURCES Eugene Callaghan, Director THE REGENTS MEMBERS Ex OFFICIO The Honorable Edwin L. Mechem ...................... Governor of New Mexico Tom Wiley ......................................... Superintendent of Public Instruction APPOINTED MEMBERS Robert W. Botts ...................................................................... Albuquerque Holm 0. Bursum, Jr. ....................................................................... Socorro Thomas M. Cramer ........................................................................ Carlsbad Frank C. DiLuzio ..................................................................... Los Alamos A. A. Kemnitz ................................................................................... Hobbs Contents Page ABSTRACT ...................................................................................................... 1 FOREWORD ................................................................................................... 2 ACKNOWLEDGMENTS ............................................................................. 3 PREVIOUS REPORTS OF CAMBRIAN CEPHALOPODS ................ 4 ADEQUATELY KNOWN CAMBRIAN CEPHALOPODS, with a revision of the Plectronoceratidae ..........................................................7 -

Octopus Insularis</Italic> As a New Marine Model for Evolutionary
© 2019. Published by The Company of Biologists Ltd | Biology Open (2019) 8, bio046086. doi:10.1242/bio.046086 RESEARCH ARTICLE Octopus insularis as a new marine model for evolutionary developmental biology Ernesto Maldonado1,*, Emma Rangel-Huerta1,2, Roberto González-Gómez3,4, Gabriel Fajardo-Alvarado3,4 and Piedad S. Morillo-Velarde4,5,* ABSTRACT of aquatic animal eggs and embryos guarantees the observation of Octopuses are intriguing organisms that, together with squids and every developmental stage using microscopy and allows detailed cuttlefishes, form the extant coleoid cephalopods. This group includes experimental analysis from the first cell division through to the many species that can potentially be used as models in the fields of formation of embryonic germ layers and organogenesis (Boletzky biomedicine, developmental biology, evolution, neuroscience and et al., 2006). Finally, small embryos allow reasonable sample sizes even for robotics research. The purpose of this work is to first to be tested together using multi-well plates to provide multiple present a simple method for maintaining Octopus insularis embryos experimental replicates at the same time, making them cost- under a laboratory setup. Second, we show that these embryos are effective animal models (Hill et al., 2005). suitable for detailed analyses of specific traits that appear during Coleoid cephalopods (octopus, squid and cuttlefish) exhibit the developmental stages, including the eyes, hearts, arms, suckers, largest nervous systems found among invertebrates (Young, 1971) chromatophores and Kölliker’s organs. Similar complex traits between and a sophisticated visual system controlling body color changes for cephalopods and vertebrates such as the visual, cardiovascular, communication, camouflage and mimicry (Hanlon et al., 2011; neural and pigmentation systems are generally considered to be a Robin et al., 2014). -

Squid Giant Axon (Glia/Neurons/Secretion)
Proc. Nat. Acad. Sci. USA Vol. 71, No. 4, pp. 1188-1192, April 1974 Transfer of Newly Synthesized Proteins from Schwann Cells to the Squid Giant Axon (glia/neurons/secretion) R. J. LASEK*, H. GAINERt, AND R. J. PRZYBYLSKI* Marine Biological Laboratory, Woods Hole, Massachusetts 02543 Communicated by Walle J. H. Nauta, November 28, 1973 ABSTRACT The squid giant axon is presented as a teins synthesized in the Schwann cells surrounding the axon model for the study of macromolecular interaction be- tween cells in the nervous system. When the isolated giant are subsequently transferred into the axoplasm. axon was incubated in sea water containing [3Hjleucine MATERIALS AND METHODS for 0.5-5 hr, newly synthesized proteins appeared in the sheath and axoplasm as demonstrated by: (i) radioautogra- Protein synthesis was studied in squid giant axons obtained phy, (ii) separation of the -sheath and axoplasm by extru- from live squid which were kept in a sea tank and used within sion, and (iii) perfusion of electrically excitable axons. hr of obtained The absence of ribosomal RNA in the axoplasm [Lasek, 48 capture. The giant axons were by decapitat- R. J. et al. (1973) Nature 244, 162-165] coupled with other ing the squid and dissecting the axons under a stream of run- evidence indicates that the labeled proteins that are found ning sea water. The axons, 4-6 cm long, were tied with thread in the axoplasm originate in the Schwann cells surrounding at both ends, removed from the mantle, and cleaned of ad- the axon. Approximately 50%70 of the newly synthesized hering connective tissue in a petri dish filled with sea water Schwann cell proteins are transferred to the giant axon. -

Effects of Temperature on Escape Jetting in the Squid Loligo Opalescens
The Journal of Experimental Biology 203, 547–557 (2000) 547 Printed in Great Britain © The Company of Biologists Limited 2000 JEB2451 EFFECTS OF TEMPERATURE ON ESCAPE JETTING IN THE SQUID LOLIGO OPALESCENS H. NEUMEISTER*,§, B. RIPLEY*, T. PREUSS§ AND W. F. GILLY‡ Hopkins Marine Station of Stanford University, Department of Biological Science, 120 Ocean View Boulevard, Pacific Grove, 93950 CA, USA *Authors have contributed equally ‡Author for correspondence (e-mail: [email protected]) §Present address: Department of Neuroscience, Albert Einstein College of Medicine, Bronx, NY 10461, USA Accepted 19 November 1999; published on WWW 17 January 2000 Summary In Loligo opalescens, a sudden visual stimulus (flash) giant and non-giant motor axons in isolated nerve–muscle elicits a stereotyped, short-latency escape response that is preparations failed to show the effects seen in vivo, i.e. controlled primarily by the giant axon system at 15 °C. We increased peak force and increased neural activity at low used this startle response as an assay to examine the effects temperature. Taken together, these results suggest that of acute temperature changes down to 6 °C on behavioral L. opalescens is able to compensate escape jetting and physiological aspects of escape jetting. In free- performance for the effects of acute temperature reduction. swimming squid, latency, distance traveled and peak A major portion of this compensation appears to occur in velocity for single escape jets all increased as temperature the central nervous system and involves alterations in the decreased. In restrained squid, intra-mantle pressure recruitment pattern of both the giant and non-giant axon transients during escape jets increased in latency, duration systems. -

Ommastrephidae 199
click for previous page Decapodiformes: Ommastrephidae 199 OMMASTREPHIDAE Flying squids iagnostic characters: Medium- to Dlarge-sized squids. Funnel locking appara- tus with a T-shaped groove. Paralarvae with fused tentacles. Arms with biserial suckers. Four rows of suckers on tentacular clubs (club dactylus with 8 sucker series in Illex). Hooks never present hooks never on arms or clubs. One of the ventral pair of arms present usually hectocotylized in males. Buccal connec- tives attach to dorsal borders of ventral arms. Gladius distinctive, slender. funnel locking apparatus with Habitat, biology, and fisheries: Oceanic and T-shaped groove neritic. This is one of the most widely distributed and conspicuous families of squids in the world. Most species are exploited commercially. Todarodes pacificus makes up the bulk of the squid landings in Japan (up to 600 000 t annually) and may comprise at least 1/2 the annual world catch of cephalopods.In various parts of the West- ern Central Atlantic, 6 species of ommastrephids currently are fished commercially or for bait, or have a potential for exploitation. Ommastrephids are powerful swimmers and some species form large schools. Some neritic species exhibit strong seasonal migrations, wherein they occur in huge numbers in inshore waters where they are accessable to fisheries activities. The large size of most species (commonly 30 to 50 cm total length and up to 120 cm total length) and the heavily mus- cled structure, make them ideal for human con- ventral view sumption. Similar families occurring in the area Onychoteuthidae: tentacular clubs with claw-like hooks; funnel locking apparatus a simple, straight groove. -

2,3-Bisphosphoglycerate (2,3-BPG)
11-cis retinal 5.4.2 achondroplasia 19.1.21 active transport, salt 3.4.19 2,3-bisphosphoglycerate 11.2.2 acid-base balance 18.3.34 activity cycle, flies 2.2.2 2,4-D herbicide 3.3.22, d1.4.23 acid coagulation cheese 15.4.36 activity rhythms, locomotor 7.4.2 2,6-D herbicide, mode of action acid growth hypothesis, plant cells actogram 7.4.2 3.2.22 3.3.22 acute mountain sickness 8.4.19 2-3 diphosphoglycerate, 2-3-DPG, acid hydrolases 15.2.36 acute neuritis 13.5.32 in RBCs 3.2.25 acid in gut 5.1.2 acute pancreatitis 9.3.24 2C fragments, selective weedkillers acid rain 13.2.10, 10.3.25, 4.2.27 acyltransferases 11.5.39 d1.4.23 1.1.15 Adams, Mikhail 12.3.39 3' end 4.3.23 acid rain and NO 14.4.18 adaptation 19.2.26, 7.2.31 3D formula of glucose d16.2.15 acid rain, effects on plants 1.1.15 adaptation, chemosensory, 3-D imaging 4.5.20 acid rain, mobilization of soil in bacteria 1.1.27 3-D models, molecular 5.3.7 aluminium 3.4.27 adaptation, frog reproduction 3-D reconstruction of cells 18.1.16 acid rain: formation 13.2.10 17.2.17 3-D shape of molecules 7.2.19 acid 1.4.16 adaptations: cereals 3.3.30 3-D shapes of proteins 6.1.31 acid-alcohol-fast bacteria 14.1.30 adaptations: sperm 10.5.2 3-phosphoglycerate 5.4.30 acidification of freshwater 1.1.15 adaptive immune response 5' end 4.3.23 acidification 3.4.27 19.4.14, 18.1.2 5-hydroxytryptamine (5-HT) 12.1.28, acidification, Al and fish deaths adaptive immunity 19.4.34, 5.1.35 3.4.27 d16.3.31, 5.5.15 6-aminopenicillanic acid 12.1.36 acidification, Al and loss of adaptive radiation 8.5.7 7-spot ladybird -

1. in Tro Duc Tion
Cephalopods of the World 1 1. INTRO DUC TION Patrizia Jereb, Clyde F.E. Roper and Michael Vecchione he increasing exploitation of finfish resources, and the commercial status. For example, this work should be useful Tdepletion of a number of major fish stocks that formerly for the ever-expanding search for development and supported industrial-scale fisheries, forces continued utilization of ‘natural products’, pharmaceuticals, etc. attention to the once-called ‘unconventional marine resources’, which include numerous species of cephalopods. The catalogue is based primarily on information available in Cephalopod catches have increased steadily in the last 40 published literature. However, yet-to-be-published reports years, from about 1 million metric tonnes in 1970 to more than and working documents also have been used when 4 million metric tonnes in 2007 (FAO, 2009). This increase appropriate, especially from geographical areas where a confirms a potential development of the fishery predicted by large body of published information and data are lacking. G.L. Voss in 1973, in his first general review of the world’s We are particularly grateful to colleagues worldwide who cephalopod resources prepared for FAO. The rapid have supplied us with fisheries information, as well as expansion of cephalopod fisheries in the decade or so bibliographies of local cephalopod literature. following the publication of Voss’s review, meant that a more comprehensive and updated compilation was required, The fishery data reported herein are taken from the FAO particularly for cephalopod fishery biologists, zoologists and official database, now available on the Worldwide web: students. The FAO Species Catalogue, ‘Cephalopods of the FISHSTAT Plus 2009. -

8 Armed Bandits; a Closer Look at Cephalopods an Educator’S Guide to the Program
8 Armed Bandits; A Closer Look at Cephalopods An Educator’s Guide to the Program Grades K-5 Program Description: This program explores the class of mollusk known as cephalopods. Cephalopods are the most intelligent group of mollusk and most of them lack a shell. The name cephalopod means “head-foot” and contains: octopus, squid, cuttlefish and nautilus. The goal of 8-armed bandits is to teach students the characteristics, defense mechanisms, and extreme intelligence of cephalopods. *Before your class visits the Oklahoma Aquarium* This guide contains information and activities for you to use both before and after your visit to the Oklahoma Aquarium. You may want to read stories about cephalopods and their abilities to the students, present information in class, or utilize some of the activities from this booklet. 1 Table of Contents 8 armed bandits abstract 3 Educator Information 4 Vocabulary 5 Internet resources and books 6 PASS/OK Science standards 7-8 Accompanying Activities Build Your Own squid (K-5) 9 How do Squid Defend Themselves? (K-5) 10 Octopus Arms (K-3) 11 Octopus Math (pre-K-K) 12 Camouflage (K-3) 13 Octopus Puppet (K-3) 14 Hidden animals (K-1) 15 Cephalopod color pages (3) (K-5) 16 Cephalopod Magic (4-5) 19 Nautilus (4-5) 20 2 8 Armed Bandits; A Closer Look at Cephalopods: Abstract Cephalopods are a class of mollusk that are highly intelligent and unlike most other mollusk, they generally lack a shell. There are 85,000 different species of mollusk; however cephalopods only contain octopi, squid, cuttlefish and nautilus. -

An Eocene Orthocone from Antarctica Shows Convergent Evolution of Internally Shelled Cephalopods
RESEARCH ARTICLE An Eocene orthocone from Antarctica shows convergent evolution of internally shelled cephalopods Larisa A. Doguzhaeva1*, Stefan Bengtson1, Marcelo A. Reguero2, Thomas MoÈrs1 1 Department of Palaeobiology, Swedish Museum of Natural History, Stockholm, Sweden, 2 Division Paleontologia de Vertebrados, Museo de La Plata, Paseo del Bosque s/n, B1900FWA, La Plata, Argentina * [email protected] a1111111111 a1111111111 a1111111111 a1111111111 Abstract a1111111111 Background The Subclass Coleoidea (Class Cephalopoda) accommodates the diverse present-day OPEN ACCESS internally shelled cephalopod mollusks (Spirula, Sepia and octopuses, squids, Vampyro- teuthis) and also extinct internally shelled cephalopods. Recent Spirula represents a unique Citation: Doguzhaeva LA, Bengtson S, Reguero MA, MoÈrs T (2017) An Eocene orthocone from coleoid retaining shell structures, a narrow marginal siphuncle and globular protoconch that Antarctica shows convergent evolution of internally signify the ancestry of the subclass Coleoidea from the Paleozoic subclass Bactritoidea. shelled cephalopods. PLoS ONE 12(3): e0172169. This hypothesis has been recently supported by newly recorded diverse bactritoid-like doi:10.1371/journal.pone.0172169 coleoids from the Carboniferous of the USA, but prior to this study no fossil cephalopod Editor: Geerat J. Vermeij, University of California, indicative of an endochochleate branch with an origin independent from subclass Bactritoi- UNITED STATES dea has been reported. Received: October 10, 2016 Accepted: January 31, 2017 Methodology/Principal findings Published: March 1, 2017 Two orthoconic conchs were recovered from the Early Eocene of Seymour Island at the tip Copyright: © 2017 Doguzhaeva et al. This is an of the Antarctic Peninsula, Antarctica. They have loosely mineralized organic-rich chitin- open access article distributed under the terms of compatible microlaminated shell walls and broadly expanded central siphuncles. -

Effect of Fmrfamide on Voltage-Dependent Currents In
bioRxiv preprint doi: https://doi.org/10.1101/2020.09.29.318691; this version posted October 1, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. A. Chrachri 1 Effect of FMRFamide on voltage-dependent currents in identified centrifugal 2 neurons of the optic lobe of the cuttlefish, Sepia officinalis 3 4 Abdesslam Chrachri 5 University of Plymouth, Dept of Biological Sciences, Drake Circus, Plymouth, PL4 6 8AA, UK and the Marine Biological Association of the UK, Citadel Hill, Plymouth 7 PL1 2PB, UK 8 Phone: 07931150796 9 Email: [email protected] 10 11 Running title: Membrane currents in centrifugal neurons 12 13 Key words: cephalopod, voltage-clamp, potassium current, calcium currents, sodium 14 current, FMRFamide. 15 16 Summary: FMRFamide modulate the ionic currents in identified centrifugal neurons 17 in the optic lobe of cuttlefish: thus, FMRFamide could play a key role in visual 18 processing of these animals. 19 - 1 - bioRxiv preprint doi: https://doi.org/10.1101/2020.09.29.318691; this version posted October 1, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. A. Chrachri 20 Abstract 21 Whole-cell patch-clamp recordings from identified centrifugal neurons of the optic 22 lobe in a slice preparation allowed the characterization of five voltage-dependent 23 currents; two outward and three inward currents. The outward currents were; the 4- 24 aminopyridine-sensitive transient potassium or A-current (IA), the TEA-sensitive 25 sustained current or delayed rectifier (IK).