Abundance and Distribution of Certain Elements In
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Some Aspects of the Geochemistry of Fluorine
SOME ASPECTS OF THE GEOCHEMISTRY OF FLUORINE by ROBERT HENRY SERAPHIM B.App.Sci., Univ. of British Columbia 1947 M.App.Sci., Univ. of British Columbia 1948 SUBMITTED IN PARTIAL FULFILLAMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY at the MASSACHUSETTS INSTITUTE OF TECHNIOLOGY 1951 Signature of Author. -. -. .*p7,- - .-. --.---.. ,Mepartment of Geology, May 11, 1951 Certified by. .. .. .. - - - - -- - -- -*--.. - - - -- A - Thesis Supervisor 0 0D C i o GS Chairman, Departmental Committee on Graduate Students I1 SOME ASPECTS OF THE GEOCHEMISTRY OF FLUORINE ROBERT HENRY SERAPHIM Submitted for the degree of Doctor of Philosophy in the Department of Geology on May 11, 1951 Abstract A spectrochemical method has been developed to determine fluorine in minerals, rocks, and soils containing .005 to 1 percent fluorine. Samples are mixed with calcium carbonate and carbon, and arced in helium atmosphere. The intensity of a Cao band which masks the very sensitive B2 group of CaF bands is thereby effectively reduced. A B2 group head at 6036 A can be used to determine as little as .005 percent fluorine, provided three identical samples are superimposed in one spectrum. A method modified after one developed previously by Ahrens (1942) has been used to determine fluorine in rocks and minerals containing more than .03 percent fluorine. Samples are arced in air, with one exposure per spectrum, so the method is more rapid and economical, though less sensitive, than the one described above. Fluorine has been determined in about three hundred specimens and samples. Averages have been calculated for the fluorine content of apatite, amphibole, biotite, muscovite, and titanite. -

A Glossary of Uranium- and Thorium-Bearing Minerals
GEOLOGICAL SURVEY CIRCULAR 74 April 1950 A GLOSSARY OF URANIUM AND THORIUM-BEARING MINERALS By Judith Weiss Frondel and Michael F1eischer UNITED STATES DEPARTMENT OF THE INTERIOR Oscar L. Chapman, Secretary GEOLOGICAL SURVEY W. E. Wrather, Director WASHINGTON. D. C. Free on application to the Director, Geological Survey, Washington 25, D. C. A GLOSSAR-Y OF URANIUM- AND THORIUM-BEARING MINERALS By Judith Weiss Fronde! and Michael Fleischer CONTENTS Introduction ••oooooooooo••••••oo•-•oo•••oo••••••••••oooo•oo••oooooo••oo•oo•oo•oooo••oooooooo•oo• 1 .A. Uranium and thorium minerals oooo oo oo ......................... oo .... oo oo oo oo oo oooooo oo 2 B. Minerals with minor amounts of uranium and thorium 000000000000000000000000.... 10 C. Minerals that should be tested for uranium and thorium ...... 00 .. 00000000000000 14 D. Minerals that are non-uranium- or non-thorium bearing, but that have been reported to contain impurities or intergrowths of uranium, thorium, or rare-earth minerals oooooo•oo ............ oo ... oo .. oooooo'""""oo" .. 0000 16 Index oo ...... oooooo•oo••••oo•oooo•oo•oooo•·~· .. •oooo•oooooooooooo•oooooo•oooooo•oooo••oo•••oooo••• 18 INTRODUCTION The U. S. ·Geological Survey has for some time been making a systematic survey of da~ pertaining to uranium and thorium minerals and to those minerals that contain trace1 or more of uranium and thorium. This survey consists of collecting authoritative chemical, optical, and X-ray diffraction data from the literature and of adding to these data, where inadequate, by work in the laboratory. The results will he reported from time to time, and the authors welcome in- formation on additional data and names. -

Zircon, Monazite and Other Minerals Used in the Production of Chemical Com- Pounds Employed in the Manufac- Ture of Lighting Apparatus
North Carolina State Library GIFT OF \*J.^. M «v* Digitized by the Internet Archive in 2013 http://archive.org/details/zirconmonaziteot25prat V C*> ttonh Carolina Stat© ^vtef <^ Raleigh NORTH CAROLINA GEOLOGICAL AND ECONOMIC SURVEY JOSEPH HYDE PRATT, State Geologist BULLETIN No. 25 Zircon, Monazite and Other Minerals Used in the Production of Chemical Com- pounds Employed in the Manufac- ture of Lighting Apparatus BY JOSEPH HYDE PRATT, Ph.D. Raleigh, N. 0. Edwards & Broughton Printing Co. State Printers and Binders 1916 GEOLOGICAL BOARD Governor Locke Craig, ex officio chairman Raleigh Frank R. Hewitt Asheville Hugh MacRae Wilmington Henry E. Fries ". Winston-Salem John Sprunt Hill Durham Joseph Hyde Pratt, State Geologist Chapel Hill ^5 LETTER OF TRANSMITTAL Chapel Hill, M". C, October 1, 1915. To His Excellency , Honorable Locke Craig, Governor of North Carolina. Sir :—I have the honor to submit herewith for publication as Bulletin 25 a report on Zircon, Monazite and other Minerals Used in the Pro- duction of Chemical Compounds Employed in the Manufacture of Light- ing Apparatus. There is a renewed interest in the deposits of these min- erals in North Carolina, and the present report takes up not only a description of the localities in which these minerals are found, but is a technical review of the development of the lighting industry. Very truly yours, Joseph Hyde Pratt, State Geologist. ±o 5 ,3 3 2 7 CONTENTS PAGE Introduction 7 Zircon 7 Sources of Zirconia 10 Zircon 10 Baddeleyite 10 Other Zirconia-bearing Minerals 11 Occurrences and localities of Zircon 13 Zircon from Henderson County, N. -

Geochemical Association of Niobium (Columbium) and Titanium and Its Geological and Economic Significance
GEOLOGICAL SURVEY CIRCULAR 225 GEOCHEMICAL ASSOCIATION OF NIOBIUM (COLUMBIUM) AND TITANIUM AND ITS GEOLOGICAL AND ECONOMIC SIGNIFICANCE By Michael Fleischer, K. J. Murata, Janet D. Fletcher, and Perry F. Narten UNITED STATES DEPARTMENT OF THE INTERIOR Oscar L. Chapman, Secretary GEOLOGICAL SURVEY W. E. Wrather, Director GEOLOGICAL SURVEY CIRCULAR 225 GEOCHEMICAL ASSOCIATION OF NIOBIUM (COLUMBIUM) AND TITANIUM AND ITS GEOLOGICAL AND ECONOMIC SIGNIFICANCE By Michael Fleischer, K. J. Murata, Janet D. Fletcher, and Perry F. Narten Washington, D. C.,1952 Free on application to the Geological Survey, Washington 25, D. C. GEOCHEMICAL ASSOCIATION OF NIOBIUM (COLUMBIUM) AND TITANIUM AND ITS GEOLOGICAL AND ECONOMIC SIGNIFICANCE CONTENTS Page Page Abstract..................................... 1 Experimental results--Continued Introduction. 1 Niobium in bauxite. 3 A cknowlE:dgments. 2 Niobium in byproducts of bauxite plants..... 7 General geochemical considerations. 2 Niobium in titanium minerals. 8 Method of spectrographic analysis . 2 Possibility of recovery of niobium from Arkansas Experimental results . 3 bauxite and from titanium minerals. 8 Niobium in igneous rocks. 3 Literature cited........ 9 TABLES Page Table l. Niobium contentofrocks reported to contain 0.01 percent or more Nb ............................... 4 2. Niobium content of Arkansas bauxites....•...................•.........•.....•..•.•. , . ... 5 3. Niobium content of products of bauxite treatment at the Hurricane Creek plant, R.eynolds Metal Co. • . • . • . • . • . • . 7 4, Analyses of titanium- and iron-rich concentrates from black sands from the Hurricane Creek plant, Reynolds Metal Co. • • . • . 8 5.. Analyses of fractions of Ti-rich concentrate..... • • . .. ...•. 8 6. Analyses of fractions of a black sand from bauxite. • . 9 7. Analyses of fractions of black sand from Wilfley table........... • . • . .. 9 8. -

Shin-Skinner January 2018 Edition
Page 1 The Shin-Skinner News Vol 57, No 1; January 2018 Che-Hanna Rock & Mineral Club, Inc. P.O. Box 142, Sayre PA 18840-0142 PURPOSE: The club was organized in 1962 in Sayre, PA OFFICERS to assemble for the purpose of studying and collecting rock, President: Bob McGuire [email protected] mineral, fossil, and shell specimens, and to develop skills in Vice-Pres: Ted Rieth [email protected] the lapidary arts. We are members of the Eastern Acting Secretary: JoAnn McGuire [email protected] Federation of Mineralogical & Lapidary Societies (EFMLS) Treasurer & member chair: Trish Benish and the American Federation of Mineralogical Societies [email protected] (AFMS). Immed. Past Pres. Inga Wells [email protected] DUES are payable to the treasurer BY January 1st of each year. After that date membership will be terminated. Make BOARD meetings are held at 6PM on odd-numbered checks payable to Che-Hanna Rock & Mineral Club, Inc. as months unless special meetings are called by the follows: $12.00 for Family; $8.00 for Subscribing Patron; president. $8.00 for Individual and Junior members (under age 17) not BOARD MEMBERS: covered by a family membership. Bruce Benish, Jeff Benish, Mary Walter MEETINGS are held at the Sayre High School (on Lockhart APPOINTED Street) at 7:00 PM in the cafeteria, the 2nd Wednesday Programs: Ted Rieth [email protected] each month, except JUNE, JULY, AUGUST, and Publicity: Hazel Remaley 570-888-7544 DECEMBER. Those meetings and events (and any [email protected] changes) will be announced in this newsletter, with location Editor: David Dick and schedule, as well as on our website [email protected] chehannarocks.com. -

Geochemistry of Niobium and Tantalum
Geochemistry of Niobium and Tantalum GEOLOGICAL SURVEY PROFESSIONAL PAPER 612 Geochemistry of Niobium and Tantalum By RAYMOND L. PARKER and MICHAEL FLEISCHER GEOLOGICAL SURVEY PROFESSIONAL PAPER 612 A review of the geochemistry of niobium and tantalum and a glossary of niobium and tantalum minerals UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1968 UNITED STATES DEPARTMENT OF THE INTERIOR STEWART L. UDALL, Secretary GEOLOGICAL SURVEY William T. Pecora, Director Library of Congress catalog-card No. GS 68-344 For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402 - Price 50 cents (paper cover) CONTENTS Page Page Abstract_ _ __-_.. _____________________ 1 Geochemical behavior Continued Introduction. _________________________ 2 Magmatic rocks Continued General geochemical considerations. _____ 2 Volcanic rock series______--____---__.__-_-__ 2. Abundance of niobium and tantalum_____ 3 Sedimentary rocks______________________________ 2. Crustal abundance-________________ 3 Deposits of niobium and tantalum.___________________ 2£ Limitations of data________________ 3 Suggestions for future work__--___-_------__-___---_- 26 Abundance in rocks._______________ 5 References, exclusive of glossary______________________ 27 Qualifying statement.__________ 5 Glossary of niobium and tantalum minerals.___________ 3C Igneous rocks_________________ 6 Part I Classification of minerals of niobium and Sedimentary rocks.____________ 10 tantalum according to chemical types_________ 31 Abundance in meteorites and tektites. 12 Part II Niobium and tantalum minerals..-_______ 32 Isomorphous substitution.______________ 13 Part III Minerals reported to contain 1-5 percent Geochemical behavior._________________ 15 niobium and tantalum_______________________ 38 Magma tic rocks ___________________ 15 Part IV Minerals in which niobium and tantalum Granitic rocks_________________ 16 have been detected in quantities less than 1 Albitized and greisenized granitic rocks. -

Kwyjibo, a REE-Enriched Iron Oxides-Copper-Gold (IOCG) Deposit, Grenville Province, Québec
Kwyjibo, a REE-enriched iron oxides-copper-gold (IOCG) deposit, Grenville Province, Québec Serge Perreault1, a and Benoit Lafrance2 1 SOQUEM Inc., 600 avenue Centrale, Val-d’Or, QC, J9P 8P1 2 Focus Graphite Inc., 138 Rue Price ouest, bureau 205, Chicoutimi, QC, G7J 1G8 a corresponding author: [email protected] Recommended citation: Perreault, S. and Lafrance, B., 2015. Kwyjibo, a REE-enriched iron oxides-copper-gold (IOCG) deposit, Grenville Province, Québec. In: Simandl, G.J. and Neetz, M., (Eds.), Symposium on Strategic and Critical Materials Proceedings, November 13-14, 2015, Victoria, British Columbia. British Columbia Ministry of Energy and Mines, British Columbia Geological Survey Paper 2015-3, pp. 139-145. 1. Introduction 80° 75° 70° 65° 60° 55° Iron oxide-copper-gold (IOCG) refers to a group of ore LABRADOR deposits that contain more than 10% low-Ti Fe oxides (mainly QUÉBEC magnetite and hematite) and have elevated Cu, Au, REE, P, U, Ag or Co (Barton, 2014). This term excludes sedimentary iron KWYJIBO deposits, magmatic iron-titanium oxide deposits, and members 50° 50° of the porphyry copper clan. They tend to be structurally or Sept-Îles stratigraphically controlled and are temporally and spatially SUPERIOR PROVINCEFront associated with intense and pervasive Na-Ca-K metasomatism. NEWFOUNDLAND They are usually magmatic-hydrothermal deposits, commonly Grenville GRENVILLE PROVINCE associated with large-scale continental A- to I-type granitic Québec NEW suites, and are found in late-or post-orogenic, extensional, BRUNSWICK shallow- to mid-crustal intracratonic, intra-arc, or back-arc 45° Ottawa Montréal 45° settings (Barton, 2014), although metamorphic-affi liated ONTARIO deposits appear to be related to compressional settings USA NOVA SCOTIA 0 200 Toronto kilometres (Corriveau, 2007; Groves et al., 2010; Barton, 2014). -

Smithsonian Miscellaneous Collections
SMITHSONIAN MISCELLANEOUS COLLECTIONS. 156 CATALOGUE MINERALS WITH THEIR FORMULAS, ETC. PREPARED FOR THE SMITHSONIAN INSTITUTION. BY T. EGLESTON. WASHINGTON: SMITHSOXIAX INSTITTTION; JUNE, 1S63. CO?(TEXTS. Advertisement iii Introduction ........... v Chemical symbols vii Systems of crystallization ........ ix Analytical table .......... xi Catalogue of minerals ......... 1 i Check list of mineraia .....,,.. 33 Alphabetical index .» o c ...... 39 Cii) ^ ADVERTISEMENT, The following Catalogue of Mineral Species has been prepared by Mr. Egleston, at the request of the Institution, for the purpose of facilitating the arranging and labelling of collections, and the conducting of exchanges, as well as of presenting in a compact form an outline of the science of mineralogy as it exists at the present day. In labelling collections it is considered important to give the chemical composition as well as the names, and hence the formulae have been added. Some doubt was at first entertained as to the system of classi- fication which ought to be adopted; but after due consideration it was concluded to make use of that followed by Professor Dana, in the last edition of his Manual of Mineralogy. "Whatever differ- ence of opinion may exist as to the best classification, the one here employed is that which will be most generally adopted in this country, on account of the almost exclusive use of Professor Dana's excellent Manual. The Institution is under obligations to Prof. Dana, Prof Brush, Dr. Genth, and other gentlemen, for their assistance in perfecting the work, and carrying it through the press. Copies of the Catalogue, printed on one side only, to be cut apart for labels, can be furnished on application. -
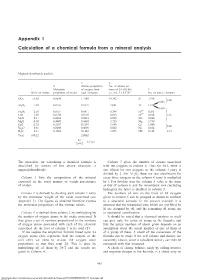
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

The Microscopic Determination of the Nonopaque Minerals
DEPARTMENT OF THE INTERIOR ALBERT B. FALL, Secretary UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, Director Bulletin 679 THE MICROSCOPIC DETERMINATION OF THE NONOPAQUE MINERALS BY ESPER S. LARSEN WASHINGTON GOVERNMENT PRINTING OFFICE 1921 CONTENTS. CHAPTER I. Introduction.................................................. 5 The immersion method of identifying minerals........................... 5 New data............................................................. 5 Need of further data.................................................... 6 Advantages of the immersion method.................................... 6 Other suggested uses for the method.................................... 7 Work and acknowledgments............................................. 7 CHAPTER II. Methods of determining the optical constants of minerals ....... 9 The chief optical constants and their interrelations....................... 9 Measurement of indices of refraction.................................... 12 The embedding method............................................ 12 The method of oblique illumination............................. 13 The method of central illumination.............................. 14 Immersion media.................................................. 14 General features............................................... 14 Piperine and iodides............................................ 16 Sulphur-selenium melts....................................... 38 Selenium and arsenic selenide melts........................... 20 Methods of standardizing -

Geological Survey
DEPARTMENT OP THE INTERIOR, BULLETIN OF THE UNITED STATES GEOLOGICAL SURVEY No. 135 WASHINGTON GOVERNMENT PRINTING OFFICE 1895 UNITED STATES GEOLOGICAL SUEVEY CHARLES D. WALCOTT, DIRECTOR THE CONSTITUTION OF THE SILICATES BY FRANK WIGGLESWORTH CLABKE CHIEF CHEMIST WASHINGTON GOVERNMENT PRINTING-OFFICE 1895 CON TE N T S. Page. Chapter I. Introduction. .................................... .............. 7 Chapter II. The theory of the silicates ....................... .............. 12 Chapter III. The orthosilicates of aluminum .................. .............. 18 I. The uephelite type .................................. .............. 18 II. The garnet-biotite type ............................'.. .............. 20 III. The feldspars and scapolites ......................... .............. 28 IV. The normal zeolites. ................................. .............. 32 V. The micas and. chlorites. ............................. .............. 45 VI. The tourmaline group ............................... .............. 56 VII. Miscellaneous species ................................ .............. 63 Chapter IV. The orthosilicates of dyad bases ................ .............. 68 Chapter V. The orthosilicates of tetrad bases ............... .............. 75 Chapter VI. The diorthosilicates ............................ .............. 81 Chapter VII. The ineta- and dimetasilicates .................. .............. 85 Chapter VIII. Summary ............................--'-..-.... .............. 101 Index ....................................................... -

The Constitution of the Natural Silicates
DEPARTMENT OF THE INTERIOR UNITED STATES GEOLOGICAL SURVEY GEORGE OTIS SMITH, DIRECTOR BULLETIN 588 THE CONSTITUTION OF THE NATURAL SILICATES BY FRANK WIGGLESWORTH CLARKE WASHINGTON GOVERNMENT PRINTING OFFICE 1914 CONTENTS. CHAPTER I. Introduction................................................ 5 CHAPTER II. The silicic acids............................................. 10 CHAPTER III. The silicates of aluminum................................... 19 General relations...................................................... 19 The nephelite type ................................................... 21 The garnet type ....................................................... 24 The feldspars and scapolites .......................................... 34 The zeolites ......................................... r ................ 40 The micas and chlorites ............................................... 51 The aluminous borosilicates .......................................... 65 Miscellaneous species ................................................ 74 CHAPTER IV. Silicates of dyad bases....................................... 87 Orthosilicates ......................................................." 87 Metasilicates ........................................................ 94 Disilicates and trisilicates ............................................ 107 CHAPTER V. Silicates of tetrad bases, titanosilicates, and columbosilicates..... 113 APPENDIX ............................................................... 124 INDEX. .................................................................