United States National Museum
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Regional Distribution of Zeolites in the Basalts of the Faroe Islands and the Significance of Zeolites As Palaeo- Temperature Indicators
The regional distribution of zeolites in the basalts of the Faroe Islands and the significance of zeolites as palaeo- temperature indicators Ole Jørgensen The first maps of the regional distribution of zeolites in the Palaeogene basalt plateau of the Faroe Islands are presented. The zeolite zones (thomsonite-chabazite, analcite, mesolite, stilbite-heulandite, laumontite) continue below sea level and reach a depth of 2200 m in the Lopra-1/1A well. Below this level, a high temperature zone occurs characterised by prehnite and pumpellyite. The stilbite-heulan- dite zone is the dominant mineral zone on the northern island, Vágar, the analcite and mesolite zones are the dominant ones on the southern islands of Sandoy and Suðuroy and the thomsonite-chabazite zone is dominant on the two northeastern islands of Viðoy and Borðoy. It is estimated that zeolitisa- tion of the basalts took place at temperatures between about 40°C and 230°C. Palaeogeothermal gradients are estimated to have been 66 ± 9°C/km in the lower basalt formation of the Lopra area of Suðuroy, the southernmost island, 63 ± 8°C/km in the middle basalt formation on the northernmost island of Vágar and 56 ± 7°C/km in the upper basalt formation on the central island of Sandoy. A linear extrapolation of the gradient from the Lopra area places the palaeosurface of the basalt plateau near to the top of the lower basalt formation. On Vágar, the palaeosurface was somewhere between 1700 m and 2020 m above the lower formation while the palaeosurface on Sandoy was between 1550 m and 1924 m above the base of the upper formation. -

Metamorphism of Sedimentary Manganese Deposits
Acta Mineralogica-Petrographica, Szeged, XX/2, 325—336, 1972. METAMORPHISM OF SEDIMENTARY MANGANESE DEPOSITS SUPRIYA ROY ABSTRACT: Metamorphosed sedimentary deposits of manganese occur extensively in India, Brazil, U. S. A., Australia, New Zealand, U. S. S. R., West and South West Africa, Madagascar and Japan. Different mineral-assemblages have been recorded from these deposits which may be classi- fied into oxide, carbonate, silicate and silicate-carbonate formations. The oxide formations are represented by lower oxides (braunite, bixbyite, hollandite, hausmannite, jacobsite, vredenburgite •etc.), the carbonate formations by rhodochrosite, kutnahorite, manganoan calcite etc., the silicate formations by spessartite, rhodonite, manganiferous amphiboles and pyroxenes, manganophyllite, piedmontite etc. and the silicate-carbonate formations by rhodochrosite, rhodonite, tephroite, spessartite etc. Pétrographie and phase-equilibia data indicate that the original bulk composition in the sediments, the reactions during metamorphism (contact and regional and the variations and effect of 02, C02, etc. with rise of temperature, control the mineralogy of the metamorphosed manga- nese formations. The general trend of formation and transformation of mineral phases in oxide, carbonate, silicate and silicate-carbonate formations during regional and contact metamorphism has, thus, been established. Sedimentary manganese formations, later modified by regional or contact metamorphism, have been reported from different parts of the world. The most important among such deposits occur in India, Brazil, U.S.A., U.S.S.R., Ghana, South and South West Africa, Madagascar, Australia, New Zealand, Great Britain, Japan etc. An attempt will be made to summarize the pertinent data on these metamorphosed sedimentary formations so as to establish the role of original bulk composition of the sediments, transformation and reaction of phases at ele- vated temperature and varying oxygen and carbon dioxide fugacities in determin- ing the mineral assemblages in these deposits. -

Download the Scanned
THn ArrERrcAN M rlrERA LocIST JOURNAL OF THE MINERALOGICAL SOCIETY OF AMERICA Vol. 50 ]ANUARY-FEBRUARY. 1965 Nos. 1 and 2 STUDIES OF THE TORBERNITE MINERALS (III): ROLE OF THE INTERLAYER OXONIUM, POTASSIUM, AND AMMONIUM IONS, AND WATER MOLECULES1 Mercorlt Ross Ar.rnH. T. EvaNs, Jn., LI. S. GeologicalSuraey, Wash,i,ngton,D. C. Alstnact Structural and chemical evidence is given to show that solid-solution series probably exist in the torbernite mineral group between the foliowing pairs of end members: K(UOzAsOa)'3I{:O (abernathyite) and H3O(UOrAsOD.3HzO (troegerite),K(UOrPO' '3HzO (meta-ankoleite) and HaO(UOzPO4).3HrO, NH+(UOzAsOd.3HzO and lroegerite, and NHr(UOzPOt '3HrO (uramphite) and H:O(UO:PO ) '3HrO. These seriesare erpressed by the formula A,_u(ttro)u(uorxo4).3H,o r,vhereA:K+ or NHa+, and X:As or P. Other possiblesolid-solution seriesare also given. The structures of K(UOzAsOa).3HzO (abernathyite), NHr(UOzAsOr)'3HzO, K(HrO)(UO:AsOa)z'6HrO, and Cu(UOrPOr)r'SHzO (meta-torbernite) are related to other torbernite minerals and possible structural similarities are suggested. The role of the interlayer cations and water moleculesin the torbernite minerals is related to other mineral groups including the zeolites, the feldspars and micas, the clay minerals, the jarosites and alunites, and the clathrate compounds. It is predicted that the distribution of the cations H3O+, K+, and NHr+ over the ll'ater molecules sites observed in the abernathyite-like compounds may be a common feature of the interlayer structure of the hydrous layer sili- cates and of the intercage structure of the zeolites and clathrate compounds The specia- nature of the oxonium ion in hvdrous structures is discussed. -

C:\Documents and Settings\Alan Smithee\My Documents\MOTM
I`mt`qx1/00Lhmdq`knesgdLnmsg9Rbnkdbhsd This month’s mineral, scolecite, is an uncommon zeolite from India. Our write-up explains its origin as a secondary mineral in volcanic host rocks, the difficulty of collecting this fragile mineral, the unusual properties of the zeolite-group minerals, and why mineralogists recently revised the system of zeolite classification and nomenclature. OVERVIEW PHYSICAL PROPERTIES Chemistry: Ca(Al2Si3O10)A3H2O Hydrous Calcium Aluminum Silicate (Hydrous Calcium Aluminosilicate), usually containing some potassium and sodium. Class: Silicates Subclass: Tectosilicates Group: Zeolites Crystal System: Monoclinic Crystal Habits: Usually as radiating sprays or clusters of thin, acicular crystals or Hairlike fibers; crystals are often flattened with tetragonal cross sections, lengthwise striations, and slanted terminations; also massive and fibrous. Twinning common. Color: Usually colorless, white, gray; rarely brown, pink, or yellow. Luster: Vitreous to silky Transparency: Transparent to translucent Streak: White Cleavage: Perfect in one direction Fracture: Uneven, brittle Hardness: 5.0-5.5 Specific Gravity: 2.16-2.40 (average 2.25) Figure 1. Scolecite. Luminescence: Often fluoresces yellow or brown in ultraviolet light. Refractive Index: 1.507-1.521 Distinctive Features and Tests: Best field-identification marks are acicular crystal habit; vitreous-to-silky luster; very low density; and association with other zeolite-group minerals, especially the closely- related minerals natrolite [Na2(Al2Si3O10)A2H2O] and mesolite [Na2Ca2(Al6Si9O30)A8H2O]. Laboratory tests are often needed to distinguish scolecite from other zeolite minerals. Dana Classification Number: 77.1.5.5 NAME The name “scolecite,” pronounced SKO-leh-site, is derived from the German Skolezit, which comes from the Greek sklx, meaning “worm,” an allusion to the tendency of its acicular crystals to curl when heated and dehydrated. -

Mineral Processing
Mineral Processing Foundations of theory and practice of minerallurgy 1st English edition JAN DRZYMALA, C. Eng., Ph.D., D.Sc. Member of the Polish Mineral Processing Society Wroclaw University of Technology 2007 Translation: J. Drzymala, A. Swatek Reviewer: A. Luszczkiewicz Published as supplied by the author ©Copyright by Jan Drzymala, Wroclaw 2007 Computer typesetting: Danuta Szyszka Cover design: Danuta Szyszka Cover photo: Sebastian Bożek Oficyna Wydawnicza Politechniki Wrocławskiej Wybrzeze Wyspianskiego 27 50-370 Wroclaw Any part of this publication can be used in any form by any means provided that the usage is acknowledged by the citation: Drzymala, J., Mineral Processing, Foundations of theory and practice of minerallurgy, Oficyna Wydawnicza PWr., 2007, www.ig.pwr.wroc.pl/minproc ISBN 978-83-7493-362-9 Contents Introduction ....................................................................................................................9 Part I Introduction to mineral processing .....................................................................13 1. From the Big Bang to mineral processing................................................................14 1.1. The formation of matter ...................................................................................14 1.2. Elementary particles.........................................................................................16 1.3. Molecules .........................................................................................................18 1.4. Solids................................................................................................................19 -

Prehistoric Exploitation of Limnosilicites in Northern Hungary: Problems and Perspectives Zsolt Mester and Norbert Faragó
Archaeologia Polona, vol. 54: 2016, 1 – 5 PL ISSN 0066 - 5924 Editorial The first scientific investigations of the sources of flint in Poland were undertaken by archaeologist Stefan Krukowski and geologist Jan Samsonowicz in the early 20th century. Krukowski used archaeological materials to identify the macroscopic char- acteristics of ‘chocolate’ flints, described their differences, and showed the potential location of the deposits (Krukowski 1920: 189–195; Budziszewski 2008: 33). In the search for deposits of flint, their outcrops, and prehistoric mines, Krukowski was accompanied by young geologist Jan Samsonowicz. The result of their cooperation was the discovery in 1921 of in situ deposits and surface accumulations of limestones containing fragments of flint and, in 1922, the identification of a prehistoric mine at Krzemionki Opatowskie (Krukowski 1923; Samsonowicz 1923; Bąbel 2014). This long tradition of studying siliceous rocks has continued at the Institute of Archaeology and Ethnology, Polish Academy of Science. In 1965 Zygmunt Krzak published the first characterization of gray white-spotted (świeciechów) flint (Krzak 1965) and five years later he described Turonian flint from Ożarów (Krzak 1970). In 1971 Romuald Schild devised a classification of ‘chocolate’ flint from the north-east margin of the Holy Cross (Świątokrzyskie) Mountains (Schild 1971, 1976) and Bogdan Balcer investigated a flint mine in Świeciechów, Kraśnik district, and the use of gray white-spotted (świeciechów) flint during the Neolithic (Balcer 1975, 1976). In 1980 Jacek Lech discussed the geology of Jurassic-Cracow flint and showed its relevance to archaeology (Lech 1980). Since that time Polish archeologists have carried out many investigations on different types of flint (e.g., Budziszewski and Michniak 1983/1989; Pawlikowski 1989; Budziszewski and Michinak eds 1995; Schild and Sulgostowska eds 1997; Matraszek and Sałaciński eds 2002; Gutowski 2004; Borkowski et al., 2008; Migaszewski et al., 2006, Krajcarz et al., 2014). -

List of New Mineral Names: with an Index of Authors
415 A (fifth) list of new mineral names: with an index of authors. 1 By L. J. S~v.scs~, M.A., F.G.S. Assistant in the ~Iineral Department of the,Brltish Museum. [Communicated June 7, 1910.] Aglaurito. R. Handmann, 1907. Zeita. Min. Geol. Stuttgart, col. i, p. 78. Orthoc]ase-felspar with a fine blue reflection forming a constituent of quartz-porphyry (Aglauritporphyr) from Teplitz, Bohemia. Named from ~,Xavpo~ ---- ~Xa&, bright. Alaito. K. A. ~Yenadkevi~, 1909. BuU. Acad. Sci. Saint-P6tersbourg, ser. 6, col. iii, p. 185 (A~am~s). Hydrate~l vanadic oxide, V205. H~O, forming blood=red, mossy growths with silky lustre. Founi] with turanite (q. v.) in thct neighbourhood of the Alai Mountains, Russian Central Asia. Alamosite. C. Palaehe and H. E. Merwin, 1909. Amer. Journ. Sci., ser. 4, col. xxvii, p. 899; Zeits. Kryst. Min., col. xlvi, p. 518. Lead recta-silicate, PbSiOs, occurring as snow-white, radially fibrous masses. Crystals are monoclinic, though apparently not isom0rphous with wol]astonite. From Alamos, Sonora, Mexico. Prepared artificially by S. Hilpert and P. Weiller, Ber. Deutsch. Chem. Ges., 1909, col. xlii, p. 2969. Aloisiite. L. Colomba, 1908. Rend. B. Accad. Lincei, Roma, set. 5, col. xvii, sere. 2, p. 233. A hydrated sub-silicate of calcium, ferrous iron, magnesium, sodium, and hydrogen, (R pp, R',), SiO,, occurring in an amorphous condition, intimately mixed with oalcinm carbonate, in a palagonite-tuff at Fort Portal, Uganda. Named in honour of H.R.H. Prince Luigi Amedeo of Savoy, Duke of Abruzzi. Aloisius or Aloysius is a Latin form of Luigi or I~ewis. -

Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite
minerals Article Thermal Annealing and Phase Transformation of Serpentine-Like Garnierite Arun Kumar 1,2 , Michele Cassetta 1, Marco Giarola 3, Marco Zanatta 4 , Monique Le Guen 5, Gian Domenico Soraru 6 and Gino Mariotto 1,* 1 Department of Computer Science, University of Verona, 37134 Verona, Italy; [email protected] (A.K.); [email protected] (M.C.) 2 CNR-Institute for Microelectronics and Microsystems, Agrate Brianza, 20864 Agrate, Italy 3 Centro Piattaforme Tecnologiche (CPT), University of Verona, 37134 Verona, Italy; [email protected] 4 Department of Physics, University of Trento, 38123 Povo, Italy; [email protected] 5 Innovation Technology Direction, ERAMET IDEAS, 78190 Trappes, France; [email protected] 6 Department of Industrial Engineering, University of Trento, 38123 Povo, Italy; [email protected] * Correspondence: [email protected] Abstract: This study is focused on the vibrational and microstructural aspects of the thermally induced transformation of serpentine-like garnierite into quartz, forsterite, and enstatite occur- ring at about 620 ◦C. Powder specimens of garnierite were annealed in static air between room temperature and 1000 ◦C. The kinetic of the transformation was investigated by means of thermo- gravimetric and differential thermal analysis, and the final product was extensively characterized via micro-Raman spectroscopy and X-ray diffraction. Our study shows that serpentine-like garnierite consists of a mixture of different mineral species. Furthermore, these garnierites and their compo- sition can provide details based on the mineralogy and the crystalline phases resulting from the thermal treatment. Citation: Kumar, A.; Cassetta, M.; Giarola, M.; Zanatta, M.; Le Guen, M.; Keywords: garnierite; phase transformation; TGA/DSC; XRD; micro-Raman spectroscopy Soraru, G.D.; Mariotto, G. -

Mixite Bicu6(Aso4)3(OH)6 • 3H2O C 2001-2005 Mineral Data Publishing, Version 1 Crystal Data: Hexagonal
Mixite BiCu6(AsO4)3(OH)6 • 3H2O c 2001-2005 Mineral Data Publishing, version 1 Crystal Data: Hexagonal. Point Group: 6/m. As acicular crystals, elongated along [0001], commonly in mats, radial fibrous aggregates, or cross-fiber veinlets. Physical Properties: Hardness = 3–4 D(meas.) = 3.79–3.83 D(calc.) = [4.04] Optical Properties: Transparent to translucent. Color: Blue-green to emerald-green, pale green, white; pale green to colorless in transmitted light. Streak: Pale bluish green. Luster: Vitreous, silky in aggregates. Optical Class: Uniaxial (+). Pleochroism: O = colorless; E = bright green. Absorption: E > O. ω = 1.743–1.749 = 1.810–1.830 Cell Data: Space Group: P 63/m. a = 13.646(2) c = 5.920(1) Z = 2 X-ray Powder Pattern: Anton mine, Germany. 12.03 (10), 2.46 (9), 3.57 (8), 2.95 (7), 2.86 (6), 2.70 (6), 2.57 (6) Chemistry: (1) (2) (3) P2O5 1.05 0.06 As2O5 29.51 28.79 29.64 SiO2 0.42 Fe2O3 0.97 Bi2O3 12.25 11.18 20.03 FeO 1.52 CuO 44.23 43.89 41.04 ZnO 2.70 CaO 0.83 0.26 H2O 11.06 11.04 9.29 Total 100.45 99.31 100.00 • (1) J´achymov, Czech Republic. (2) Tintic district, Utah, USA. (3) BiCu6(AsO4)3(OH)6 3H2O. Mineral Group: Mixite group. Occurrence: An uncommon secondary mineral in the oxidized zone of copper deposits. Association: Bismutite, smaltite, bismuth, atelestite, erythrite, malachite, barite. Distribution: From the Geister vein, Werner mine, J´achymov (Joachimsthal), Czech Republic. -

Some Aspects of the Geochemistry of Fluorine
SOME ASPECTS OF THE GEOCHEMISTRY OF FLUORINE by ROBERT HENRY SERAPHIM B.App.Sci., Univ. of British Columbia 1947 M.App.Sci., Univ. of British Columbia 1948 SUBMITTED IN PARTIAL FULFILLAMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY at the MASSACHUSETTS INSTITUTE OF TECHNIOLOGY 1951 Signature of Author. -. -. .*p7,- - .-. --.---.. ,Mepartment of Geology, May 11, 1951 Certified by. .. .. .. - - - - -- - -- -*--.. - - - -- A - Thesis Supervisor 0 0D C i o GS Chairman, Departmental Committee on Graduate Students I1 SOME ASPECTS OF THE GEOCHEMISTRY OF FLUORINE ROBERT HENRY SERAPHIM Submitted for the degree of Doctor of Philosophy in the Department of Geology on May 11, 1951 Abstract A spectrochemical method has been developed to determine fluorine in minerals, rocks, and soils containing .005 to 1 percent fluorine. Samples are mixed with calcium carbonate and carbon, and arced in helium atmosphere. The intensity of a Cao band which masks the very sensitive B2 group of CaF bands is thereby effectively reduced. A B2 group head at 6036 A can be used to determine as little as .005 percent fluorine, provided three identical samples are superimposed in one spectrum. A method modified after one developed previously by Ahrens (1942) has been used to determine fluorine in rocks and minerals containing more than .03 percent fluorine. Samples are arced in air, with one exposure per spectrum, so the method is more rapid and economical, though less sensitive, than the one described above. Fluorine has been determined in about three hundred specimens and samples. Averages have been calculated for the fluorine content of apatite, amphibole, biotite, muscovite, and titanite. -
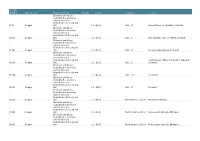
BRSUG Number Mineral Name Hey Index Group Hey No
BRSUG Number Mineral name Hey Index Group Hey No. Chem. Country Locality Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-37 Copper Au) 1.1 4[Cu] U.K., 17 Basset Mines, nr. Redruth, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-151 Copper Au) 1.1 4[Cu] U.K., 17 Phoenix mine, Cheese Wring, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-280 Copper Au) 1.1 4[Cu] U.K., 17 County Bridge Quarry, Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and South Caradon Mine, 4 miles N of Liskeard, B-319 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-394 Copper Au) 1.1 4[Cu] U.K., 17 ? Cornwall? Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-395 Copper Au) 1.1 4[Cu] U.K., 17 Cornwall Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-539 Copper Au) 1.1 4[Cu] North America, U.S.A Houghton, Michigan Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-540 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, Ag and B-541 Copper Au) 1.1 4[Cu] North America, U.S.A Keweenaw Peninsula, Michigan, Elements and Alloys (including the arsenides, antimonides and bismuthides of Cu, -

Infrared and Raman Spectroscopic Characterization of the Carbonate Bear- Ing Silicate Mineral Aerinite - Implications for the Molecular Structure
This may be the author’s version of a work that was submitted/accepted for publication in the following source: Frost, Ray, Scholz, Ricardo, & Lopez Toro, Andres (2015) Infrared and Raman spectroscopic characterization of the carbonate bear- ing silicate mineral aerinite - Implications for the molecular structure. Journal of Molecular Structure, 1097, pp. 1-5. This file was downloaded from: https://eprints.qut.edu.au/84503/ c Consult author(s) regarding copyright matters This work is covered by copyright. Unless the document is being made available under a Creative Commons Licence, you must assume that re-use is limited to personal use and that permission from the copyright owner must be obtained for all other uses. If the docu- ment is available under a Creative Commons License (or other specified license) then refer to the Licence for details of permitted re-use. It is a condition of access that users recog- nise and abide by the legal requirements associated with these rights. If you believe that this work infringes copyright please provide details by email to [email protected] License: Creative Commons: Attribution-Noncommercial-No Derivative Works 2.5 Notice: Please note that this document may not be the Version of Record (i.e. published version) of the work. Author manuscript versions (as Sub- mitted for peer review or as Accepted for publication after peer review) can be identified by an absence of publisher branding and/or typeset appear- ance. If there is any doubt, please refer to the published source. https://doi.org/10.1016/j.molstruc.2015.05.008 Infrared and Raman spectroscopic characterization of the carbonate bearing silicate mineral aerinite – implications for the molecular structure Ray L.