Appendix C: Sensor Packaging and Installation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

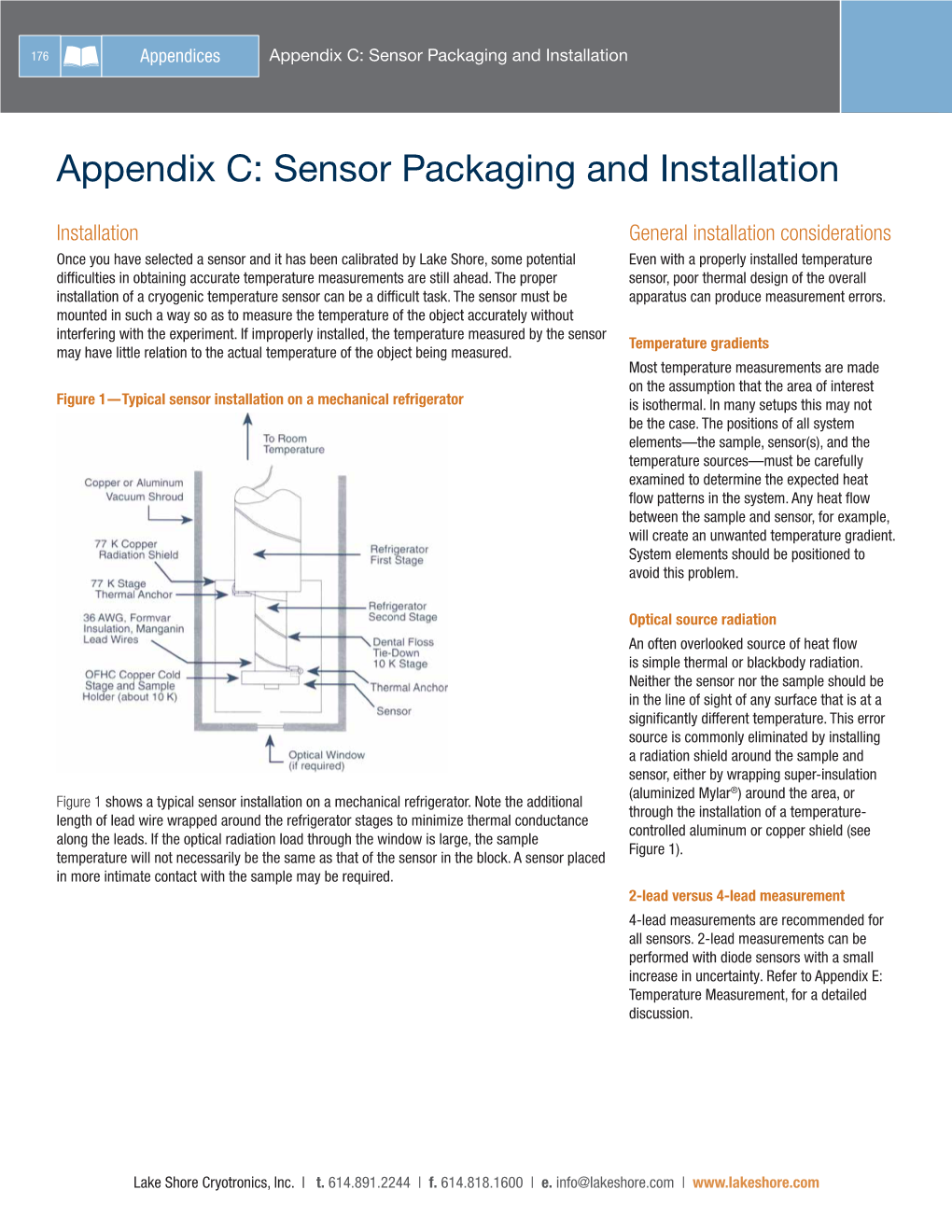
Sensor Connectors
Sensor Connectors Thermocouple Connector - Two Pole Assembly Termination Code: 2 • Glass filled thermoplastic body provides high strength at temperatures up to 425°F 218°C) as well as low moisture absorption and good dielectric constant . • Heavy duty hollow pin construction prevents reverse mating of polarity.* • Body color coded to ISA and ANSI standards. 1” • Polarity indicated by symbols molded into body. • Contacts made of thermocouple materials which meet ISA and ANSI standards . • Jack spring loaded to insure firm grip to plug. • Accepts wire sizes to 14 awg. • Single screw cover cap for fast assembly. • Accepts crimp and tube adapter for product from .020 to .375. • Finger grips to permit ease of connection. 7/16” • Quick wiring hook up with large head screws and wire channel. Catalog Number Thermocouple Body Actual Alloy Plugs Jacks Type Color + In Connector - 1/2” LP-J L J-J Iron-Constantan® Black Iron Constantan® LP-K L J-K Chromel®-Alumel® Yellow Chromel® Alumel® 7/8” LP-E L J-E Chromel®-Constantan® Violet Chromel® Constantan® LP-T L J-T Copper-Constantan® Blue Copper Constantan® 1 3/8” LP-R/S L J-R/S Platinum/Rhodium- Green Copper #11 Alloy Platinum LP-CU L J-CU Uncompensated White Copper Copper Assembly Termination Code: 5 *Solid pin available on above construction. Add S to Part No. (i.e. LPS-J) Miniature Thermocouple Connector Assembly Termination Code: 3 • Thermoplastic body provides high strength at temperatures up to 425°F (218°C) as well as low moisture absorption and good dielectric constant . • Small, light weight and space saving. -

THERMO POWER of Kr IMPLANTED MANGANIN- Cu THERMOCOUPLE T
Solid State Physics ANNUAL REPORT 2005 THERMO POWER OF Kr IMPLANTED MANGANIN- Cu THERMOCOUPLE T. WilczyĔ ska, R. WiĞ niewski, K. Wieteska Institute of Atomic Energy Low electric thermo power of manganin (Cu- junctions were placed into small glass containers insu- Mn_Ni alloy) relative to the copper is an advantage of lated thermally from each other by polystyrene box. The manganin alloy. The aim of this investigation was to hot junction container had suitable electrical heater. check if electric thermo power remains low after im- Temperature of the junctions was measured with mer- plantation with Kr ions. The work described is part of cury micro thermometer or an additional constantan- wide projected concerned with studies on properties of copper thermocouple. manganin implanted with various heavy ions [1]. Our measurements revealed that implantation of The 10P m thick stripe samples of 1 × 75mm were manganin with Kr ions reduces its thermo power rela- implanted with Kr ions in Laboratory of Nuclear Reac- tive to copper by ~50%. Another result of our study is tion at Joint Institute for Nuclear Research, Dubna. A that thermo power of non implanted manganin relative moderation aluminum foil of 11P m thick and 3mm to Cu is a little lower than given in literature. width was used. According to modeling performed with To assess the origin of such significant decrease in TRIM code [2] implantation produced the enhanced thermo EMF after implantation the thermally forced deposition layer near the back side of sample (Fig. 1). electron diffusion in junctions, contact potential and After implantation the samples were annealed for 100h o phonon forced electron drag components of the Seebeck at 130 - 150 C. -

Strain Gage Technical Data
STRAIN GAGE TECHNICAL DATA STRAIN GAGE MEASUREMENT The most universal measuring device for the electrical measurement of mechanical quantities is the strain gage. Several types of strain gages depend for their operation on the proportional variance of electrical resistance to strain: the piezoresistive or semi-conductor gage, the carbon resistive gage, the bonded metallic wire, and foil resistance gages. The bonded resistance strain gage is by far the most widely used in experimental stress analysis. This gage consists of a grid of very fine wire or foil bonded to a backing or carrier matrix. The electrical resistance of the grid varies linearly with strain. In use, the carrier matrix is bonded to the surface, force is applied, and the strain is found by measuring the change in resistance. The bonded resistance strain gage is low in cost, can be made with a short gage length, is only moderately affected by the bridge. This method assumes a wiring is located in a time-varying temperature changes, has small linear relationship between voltage magnetic field. out and strain, an initially balanced physical size and low mass, and Magnetic induction can be has fairly high sensitivity to strain. bridge, and a known VIN. In reality, the VOUT-strain relationship is controlled by using twisted lead In a strain gage application, the wires and forming minimum but carrier matrix and the adhesive nonlinear, but for strains up to a few thousand micro-strain, the error is equal loop areas in each side of must work together to transmit the the bridge. strain from the specimen to the grid. -

Chapter 25 Resistance and Current
Chapter 25 Resistance and Current Current in Wires • We define the Ampere (amp) to be one Coulomb of charge flow per second • A Coulomb is about 7 x 1018 electrons (or protons) of charge • For reference a “mole” is about 6.02 x 1023 units • Thus a “mole” of Copper 63.5 g/mole (z=29, A=63 (69.15% - 34 Neutrons, A=65 ( 30.85% - 36 Neutrons ) • Contains about 3 x 106 Coulombs BUT only outer electrons are free to move (4S1 state) – one electron per Cu atom in “valence band” • Density of Copper is about 8.9 g/cm3 • Density of free electrons in Cu ~ 1.4 x 104 Coul/cm3 • Or density of free electrons ~ 1023 e/cm3 A bit of History • chalkos (χαλκός) in Greek • Cyprium in Roman times as it was found in Cyprus • This was simplified to Cuprum in Latin and then • Copper in English • Copper mined in what is now Wisconsin 6000-3000 BCE • Copper plumbing found in Egyptian pyramid 3000 BCE • Small amount of Tin (Sn) helps in casting – Bronze (Cu-Sn) Ancient mine in Timna Valley – Negev Israel Current in wire • Lets assume a metal wire has n free charges/ vol • Assume the wire has cross sectional area A • Assume the charges (electrons) move at “drift speed” vd • Lets follow a section of charge q in length x • q = n*A*x (n*volume)e • Where e = electron charge • This volume move (drifts) at speed vd • This charge moves thru x in time • t = x/vd • The current is I= q/t = n*A*x*e/ (x/vd ) = nAvde , Wire gauges AWG – American Wire Gauge • Larger wire gauge numbers are smaller size wire • By definition 36 gauge = 0.005 inches diam • By definition 0000 gauge “4 -

Linear Thermal Expansion Coefficients of Metals and Alloys
17 Material Expansion Coefficients Chapter 17 Material Expansion Coefficients Linear Thermal Expansion Coefficients of Metals and Alloys Linear Thermal Expansion Coefficients of Metals and Alloys Table 17-1 provides the linear thermal expansion coefficients of the most frequently used metals and allows. Table 17-1. Linear thermal expansison coefficients of metals and alloys Coefficient of Expansion Alloys ppm/°C ppm/°F ALUMINUM AND ALUMINUM ALLOYS Aluminum (99.996%) 23.6 13.1 Wrought Alloys EC 1060 and 1100 23.6 13.1 2011 and 2014 23.0 12.8 2024 22.8 12.7 2218 22.3 12.4 3003 23.2 12.9 4032 19.4 10.8 5005, 5050, and 5052 23.8 13.3 5056 24.1 13.4 5083 23.4 13.0 5086 23.9 13.3 5154 23.9 13.3 5357 23.7 13.2 5456 23.9 13.3 6061 and 6063 23.4 13.0 6101 and 6151 23.0 12.8 7075 23.2 12.9 7090 and 7178 23.4 13.0 17-2 User’s Manual Chapter 17 Material Expansion Coefficients Linear Thermal Expansion Coefficients of Metals and Alloys Table 17-1. Linear thermal expansison coefficients of metals and alloys (Cont.) Coefficient of Expansion Alloys ppm/°Cppm/°F ALUMINUM AND ALUMINUM ALLOYS (Continued) Casting Alloys A13 20.4 11.4 43 and 108 22.0 12.3 A108 21.5 12.0 A132 19.0 10.6 D132 20.5 11.4 F132 20.7 11.5 138 21.4 11.9 142 22.5 12.5 195 23.0 12.8 B195 22.0 12.3 214 24.0 13.4 220 25.0 13.9 319 21.5 12.0 355 22.0 12.3 356 21.5 12.0 360 21.0 11.7 750 23.1 12.9 40E 24.7 13.8 COPPER AND COPPER ALLOYS Wrought Coppers Pure Copper 16.5 9.2 Electrolytic Tough Pitch Copper (ETP) 16.8 9.4 Deoxidized Copper, High Residual Phosphorous (DHP) 17.7 9.9 Oxygen-Free Copper 17.7 9.9 Free-Machining Copper 0.5% Te or 1% Pb 17.7 9.9 User’s Manual 17-3 Chapter 17 Material Expansion Coefficients Linear Thermal Expansion Coefficients of Metals and Alloys Table 17-1. -

Standard Cells: Their Construction, Maintenance, and Characteristics
NBS MONOGRAPH 84 Standard Cells Their Construction, Maintenance, And Characteristics U.S. DEPARTMENT OF COMMERCE NATIONAL BUREAU OF STANDARDS THE NATIONAL BUREAU OF STANDARDS The National Bureau of Standards is a principal focal point in the Federal Government for assur- ing maxinmm application of the physical and engineering sciences to the advancement of technology in industry and commerce. Us responsibilities include development and maintenance of the national standards of measurement, and the provisions of means for making measurements consistent with tliose standards; determination of physical constants and properties of materials; development of methods for testing materials, meciianisms, and structures, and making such tests as may be neces- sary, particularly for government agencies; cooperation in the establishment of standard practices for incorporation in codes and specifications; advisory service to government agencies on scientific and technical problems; invention and development of devices to serve special needs of the Government; assistance to industry, business, and consumers in the development and acceptance of commercial standards and simplified trade practice recommendations; administration of programs in cooperation w ith Lnited States business groups and standards organizations for the development of international standards of practice: and maintenance of a clearinghouse for the collection and dissemination of scientific, technical, and engineering information. The scope of the Bureau's activities is suggested in the following listing of its four Institutes and their organizational units. Institute for Basic Standards. Electricity. Metrology. Heat. Radiation Physics. Me- chanics. Applied Mathematics. Atomic Physics. Physical Chemistry. Laboratory Astrophysics.* Radio Standards Laboratory: Radio Standards Physics; Radio Standards Engineering.** Office of Standard Reference Data. Institute for -Materials Research. -

Abstract Instrument to Measure the Heat Capacity
ABSTRACT INSTRUMENT TO MEASURE THE HEAT CAPACITY OF SMALL SAMPLES A calorimeter is used to make measurements of the internal energy of a material in order to probe its thermodynamic properties, such as crystalline lattice stiffness, electronic effective mass, phase transitions, and entropy. Rare-earth metallic compounds are of interest in our lab because they are known to exhibit strongly correlated electron behavior, which gives rise to interesting phenomenon such as conventional and unconventional superconductivity, metal-insulator transitions, magnetism, and the magnetocaloric effect. The temperature dependence of specific heat is an important quantity to investigate of these materials. With limited space of our cryogenic system, we are unable to construct a calorimeter that is perfectly thermally isolated; therefore, the use of a thermal relaxation method for our heat capacity measurements will be used. A discussion on the construction of a calorimeter and implementation of the relaxation calorimetery method will be presented. Ulises Ivan Urbina August 2010 INSTRUMENT TO MEASURE THE HEAT CAPACITY OF SMALL SAMPLES by Ulises Ivan Urbina A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Physics in the College of Science and Mathematics California State University, Fresno August 2010 APPROVED For the Department of Physics: We, the undersigned, certify that the thesis of the following student meets the required standards of scholarship, format, and style of the university and the student's -

Accessories 143143
Wire IntroductionAccessories 143143 Wire Abbreviations used in this section American wire gauge AWG Single lead wire SL Duo-Twist™ wire DT Quad-Twist™ wire QT Quad-Lead™ wire QL Specifications Phosphor bronze Copper Nichrome Manganin Melting range 1223 K to 1323 K 1356 K 1673 K 1293 K Coefficient of thermal expansion 1.78 × 10-5 20 × 10-6 — 19 × 10-6 Chemical composition (nominal) 94.8% copper, 5% tin, 0.2% — 80% nickel, 20% chromium 83% copper, 13% manganese, phosphorus 4% nickel Electrical resistivity 11 µΩ·cm 1.7 µΩ·cm 120 µΩ·cm 48 µΩ·cm (at 293 K) Thermal 0.1 K NA 9 NA 0.006 conductivity 0.4 K NA 30 NA 0.02 (W/(m·K)) 1 K 0.22 70 NA 0.06 4 K 1.6 300 0.25 0.5 10 K 4.6 700 0.7 2 20 K 10 1100 2.6 3.3 80 K 25 600 8 13 150 K 34 410 9.5 16 300 K 48 400 12 22 AWG Resistance (Ω/m) Diameter Fuse Fuse current Number Name Insulated Insulation type Insulation Insulation (mm) current vacuum (A) of leads diameter thermal breakdown 4.2 K 77 K 305 K air (A) (mm) rating (K) voltage (VDC) Phosphor 1 SL-32 0.241 Polyimide bronze 2 DT-32 0.241 Polyimide 32 3.34 3.45 4.02 0.203 4.2 3.1 493 400 QT-32 0.241 Polyimide 4 QL-32 0.241 Polyimide 1 SL-36 0.152 Formvar® 368 250 2 DT-36 0.152 Polyimide 493 400 36 8.56 8.83 10.3 0.127 2.6 1.4 QT-36 0.152 Formvar® 368 250 4 QL-36 0.152 Polyimide 493 400 Nichrome 32 33.2 33.4 34 0.203 2.5 1.8 1 NC-32 0.241 Polyimide 493 400 Copper 30 0.003 0.04 0.32 0.254 10.2 8.8 1 HD-30 0.635 Teflon® 473 250 34 0.0076 0.101 0.81 0.160 5.1 4.4 2 CT-34 0.254 Teflon® 473 100 Manganin 30 8.64 9.13 9.69 0.254 4.6 4.3 1 MW-30 0.295 Heavy Formvar® 400 32 13.5 14.3 15.1 0.203 3.8 3.5 1 MW-32 0.241 Heavy Formvar® 378 400 36 34.6 36.5 38.8 0.127 2.6 2.5 1 MW-36 0.152 Heavy Formvar® 250 Lake Shore Cryotronics, Inc. -

Strain Gage Selection: Criteria, Procedures, Recommendations
MICRO-MEASUREMENTS Strain Gages and Instruments Tech Note TN-505-6 Strain Gage Selection: Criteria, Procedures, Recommendations 1.0 Introduction It must be appreciated that the process of gage selection generally involves compromises. This is because parameter The initial step in preparing for any strain gage installation choices which tend to satisfy one of the constraints or is the selection of the appropriate gage for the task. It might requirements may work against satisfying others. For at first appear that gage selection is a simple exercise, of no example, in the case of a small-radius fillet, where the great consequence to the stress analyst; but quite the opposite space available for gage installation is very limited, and is true. Careful, rational selection of gage characteristics the strain gradient extremely high, one of the shortest and parameters can be very important in: optimizing available gages might be the obvious choice. At the the gage performance for specified environmental and same time, however, gages shorter than about 0.125 in operating conditions, obtaining accurate and reliable strain (3 mm) are generally characterized by lower maximum measurements, contributing to the ease of installation, and elongation, reduced fatigue life, less stable behavior, and minimizing the total cost of the gage installation. greater installation difficulty. Another situation which often The installation and operating characteristics of a strain influences gage selection, and leads to compromise, is the gage are affected by the following parameters, which are stock of gages at hand for day-to-day strain measurements. selectable in varying degrees: While compromises are almost always necessary, the stress analyst should be fully aware of the effects of such • strain-sensitive alloy compromises on meeting the requirements of the gage • backing material (carrier) installation. -

Properties of Some Metals and Alloys
Properties of Some Metals and Alloys COPPER AND COPPER ALLOYS • WHITE METALS AND ALLOYS • ALUMINUM AND ALLOYS • MAGNESIUM ALLOYS • TITANIUM ALLOYS • RESISTANCE HEATING ALLOYS • MAGNETIC ALLOYS • CON- TROLLED EXPANSION AND CON- STANT — MODULUS ALLOYS • NICKEL AND ALLOYS • MONEL* NICKEL- COPPER ALLOYS • INCOLOY* NICKEL- IRON-CHROMIUM ALLOYS • INCONEL* NICKEL-CHROMIUM-IRON ALLOYS • NIMONIC* NICKEL-CHROMIUM ALLOYS • HASTELLOY* ALLOYS • CHLORIMET* ALLOYS • ILLIUM* ALLOYS • HIGH TEMPERATURE-HIGH STRENGTH ALLOYS • IRON AND STEEL ALLOYS • CAST IRON ALLOYS • WROUGHT STAINLESS STEEL • CAST CORROSION AND HEAT RESISTANT ALLOYS* REFRACTORY METALS AND ALLOYS • PRECIOUS METALS Copyright 1982, The International Nickel Company, Inc. Properties of Some Metals INTRODUCTION The information assembled in this publication has and Alloys been obtained from various sources. The chemical compositions and the mechanical and physical proper- ties are typical for the metals and alloys listed. The sources that have been most helpful are the metal and alloy producers, ALLOY DIGEST, WOLDMAN’S ENGI- NEERING ALLOYS, International Nickel’s publications and UNIFIED NUMBERING SYSTEM for METALS and ALLOYS. These data are presented to facilitate general compari- son and are not intended for specification or design purposes. Variations from these typical values can be expected and will be dependent upon mill practice and material form and size. Strength is generally higher, and ductility correspondingly lower, in the smaller sizes of rods and bars and in cold-drawn wire; the converse is true for the larger sizes. In the case of carbon, alloy and hardenable stainless steels, mechanical proper- ties and hardnesses vary widely with the particular heat treatment used. REFERENCES Many of the alloys listed in this publication are marketed under well-known trademarks of their pro- ducers, and an effort has been made to associate such trademarks with the applicable materials listed herein. -

Manganina 43 Resistance Heating Wire and Resistance Wire Datasheet
MANGANINA 43 RESISTANCE HEATING WIRE AND RESISTANCE WIRE DATASHEET Manganina 43 is a copper-manganese-nickel alloy (CuMnNi alloy) for use at room temperature. The alloy is characterized by very low thermal electromotive force (emf) compared to copper. Manganina 43 is typically used for the manufacturing of resistance standards, precision wire wound resistors, potentiometers, shunts and other electrical and electronic components. The alloy's low emf vs. copper makes it ideal for use in electrical circuits, especially D.C., where a spurious thermal emf could cause malfunctioning of electronic equipment. Due to the low operating temperature, the temperature coefficient of resistance is controlled to be low over a range of 15 to 35°C (59 to 95°F) CHEMICAL COMPOSITION Ni % Mn % Cu % Nominal composition 4.0 11.0 Bal. MECHANICAL PROPERTIES Wire size Yield Strength Tensile Strength Elongation Hardness Ø Rp0.2 Rm A mm (in) MPa (ksi) MPa (ksi) % Hv 1.00 (0.04) 180 (26) 390 (57) 30 110 PHYSICAL PROPERTIES Density g/cm3 (lb/in3) 8.4 (0.303) Electrical resistivity at 20°C Ω mm2/m (Ω circ. mil/ft) 0.43 (259) Temperature coefficient of resistance (15 - 35 °C) (x 10-6/K) 0 ± 15 COEFFICIENT OF THERMAL EXPANSION Temperature °C (°F) Thermal Expansion x 10-6/K (10-6 /°F) 20 - 100 (68-212) 18 (10) THERMAL CONDUCTIVITY Datasheet updated 2/4/2021 1:31:17 PM (supersedes all previous editions) 1 MANGANINA 43 Temperature °C (°F) 20 (68) W m-1 K-1 (Btu h-1ft-1°F-1) 22 (12.7) SPECIFIC HEAT CAPACITY Temperature °C (°F) 20 (68) kJ kg-1 K-1 (Btu lb-1 °F-1) 0.410 (0.10) Melting point °C (°F) 1020 (1868) Max continuous operating temperature in air °C Room temperature Magnetic properties The material is non-magnetic Disclaimer: Recommendations are for guidance only, and the suitability of a material for a specific application can be confirmed only when we know the actual service conditions. -

1.2 Low Temperature Properties of Materials
1.2 Low Temperature Properties of Materials Materials properties affect the performance of cryogenic systems. Properties of materials vary considerably with temperature Thermal Properties: Heat Capacity (internal energy), Thermal Expansion Transport Properties: Thermal conductivity, Electrical conductivity Mechanical Properties: Strength, modulus or compressibility, ductility, toughness Superconductivity Many of the materials properties have been recorded and models exist to understand and characterize their behavior Physical models Property data bases (Cryocomp®) NIST: www.cryogenics.nist.gov/MPropsMAY/material%20properties.htm What are the cryogenic engineering problems that involve materials? USPAS Cryogenics Short Course Boston, MA 6/14 to 6/18/2010 1 Cooldown of a solid component Cryogenics involves cooling things to low temperature. Therefore one needs to understand the process. If the mass and type of the object and its material are known, then the m Ti = 300 K heat content at the designated temperatures can be calculated by integrating 1st Law. ~ 0 dQ Tds= = dE+ pdv The heat removed from the component is equal to its change of T = 80 K m f internal energy, ⎛ Ti ⎞ EΔ m = ⎜ CdT⎟ ⎜ ∫ ⎟ ⎝ T f ⎠ Liquid nitrogen @ 77 K USPAS Cryogenics Short Course Boston, MA 6/14 to 6/18/2010 2 Heat Capacity of Solids C(T) General characteristics: The heat capacity is defined as the change in the heat content with temperature. The heat capacity at constant volume is, 0 300 ∂E ∂s T(K) Cv = and at constant pressure,CTp = ∂T v ∂T p 3rd Law: C 0 as T 0 rms of the heat capacity are oThese two f related through the following thermodynamic relation, 2 ∂v ⎞ ∂p ⎞ Tvβ 2 1 ∂v ⎞ 1 ∂v ⎞ CCT− = − = κ= − ⎟ β= − ⎟ p v ⎟ ⎟ v ∂p ⎟ v ∂T ∂T ⎠p∂v ⎠ T κ ⎠T ⎠ p Isothermal Volume Note: C –C is small except for p v compressibility expansivity gases, where ~ R = 8.31 J/mole K.