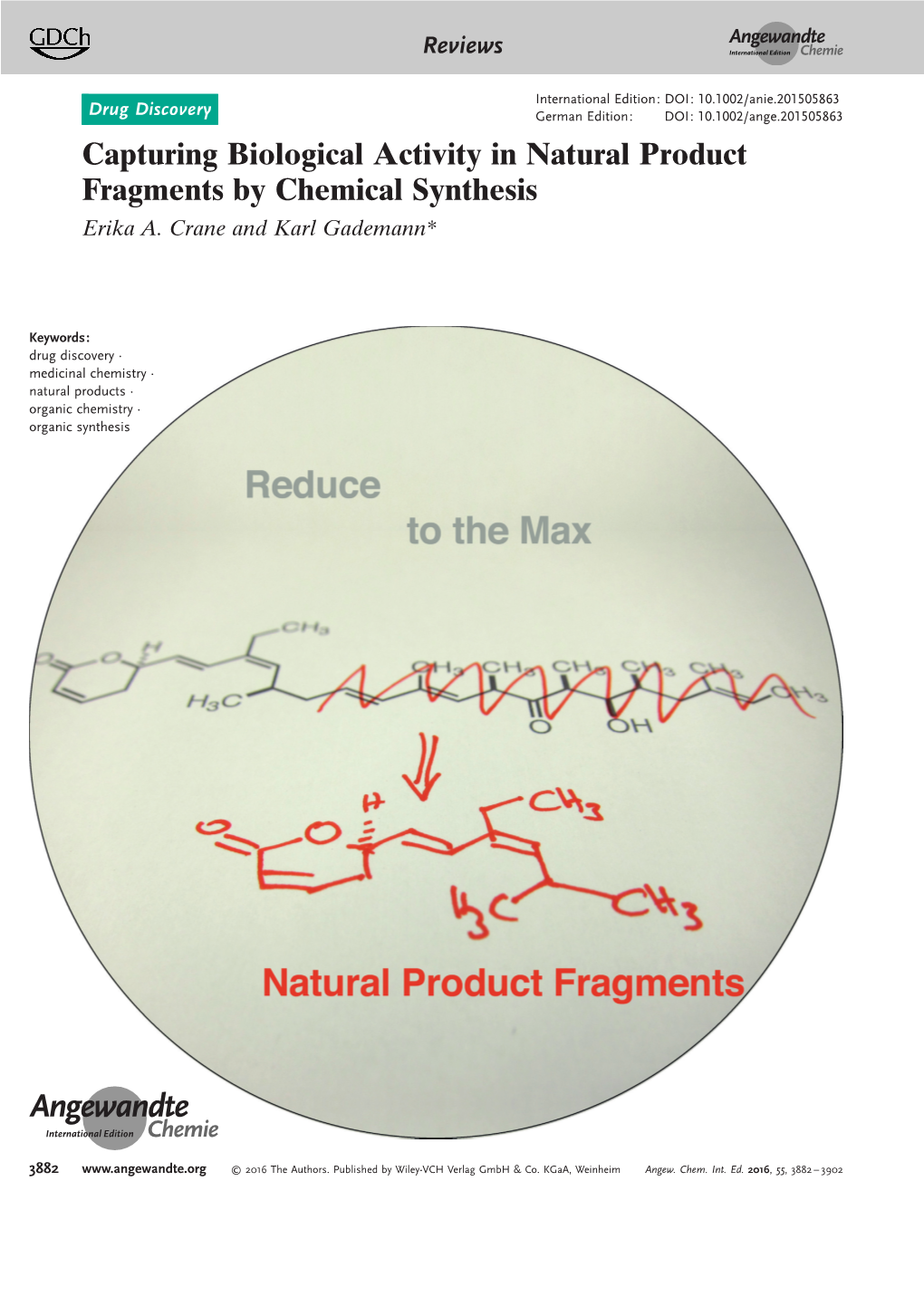
Capturing Biological Activity in Natural Product Fragments by Chemical Synthesis Erika A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Enediynes, Enyneallenes, Their Reactions, and Beyond
Advanced Review Enediynes, enyne-allenes, their reactions, and beyond Elfi Kraka∗ and Dieter Cremer Enediynes undergo a Bergman cyclization reaction to form the labile 1,4-didehy- drobenzene (p-benzyne) biradical. The energetics of this reaction and the related Schreiner–Pascal reaction as well as that of the Myers–Saito and Schmittel reac- tions of enyne-allenes are discussed on the basis of a variety of quantum chemical and available experimental results. The computational investigation of enediynes has been beneficial for both experimentalists and theoreticians because it has led to new synthetic challenges and new computational methodologies. The accurate description of biradicals has been one of the results of this mutual fertilization. Other results have been the computer-assisted drug design of new antitumor antibiotics based on the biological activity of natural enediynes, the investigation of hetero- and metallo-enediynes, the use of enediynes in chemical synthesis and C materials science, or an understanding of catalyzed enediyne reactions. " 2013 John Wiley & Sons, Ltd. How to cite this article: WIREs Comput Mol Sci 2013. doi: 10.1002/wcms.1174 INTRODUCTION symmetry-allowed pericyclic reactions, (ii) aromatic- ity as a driving force for chemical reactions, and (iii) review on the enediynes is necessarily an ac- the investigation of labile intermediates with biradical count of intense and successful interdisciplinary A character. The henceforth called Bergman cyclization interactions of very different fields in chemistry provided deeper insight into the electronic structure involving among others organic chemistry, matrix of biradical intermediates, the mechanism of organic isolation spectroscopy, quantum chemistry, biochem- reactions, and orbital symmetry rules. -

WO 2018/064165 A2 (.Pdf)
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/064165 A2 05 April 2018 (05.04.2018) W !P O PCT (51) International Patent Classification: Published: A61K 35/74 (20 15.0 1) C12N 1/21 (2006 .01) — without international search report and to be republished (21) International Application Number: upon receipt of that report (Rule 48.2(g)) PCT/US2017/053717 — with sequence listing part of description (Rule 5.2(a)) (22) International Filing Date: 27 September 2017 (27.09.2017) (25) Filing Language: English (26) Publication Langi English (30) Priority Data: 62/400,372 27 September 2016 (27.09.2016) US 62/508,885 19 May 2017 (19.05.2017) US 62/557,566 12 September 2017 (12.09.2017) US (71) Applicant: BOARD OF REGENTS, THE UNIVERSI¬ TY OF TEXAS SYSTEM [US/US]; 210 West 7th St., Austin, TX 78701 (US). (72) Inventors: WARGO, Jennifer; 1814 Bissonnet St., Hous ton, TX 77005 (US). GOPALAKRISHNAN, Vanch- eswaran; 7900 Cambridge, Apt. 10-lb, Houston, TX 77054 (US). (74) Agent: BYRD, Marshall, P.; Parker Highlander PLLC, 1120 S. Capital Of Texas Highway, Bldg. One, Suite 200, Austin, TX 78746 (US). (81) Designated States (unless otherwise indicated, for every kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. -

Isolation and Identification of Cyclic Polyketides From
ISOLATION AND IDENTIFICATION OF CYCLIC POLYKETIDES FROM ENDIANDRA KINGIANA GAMBLE (LAURACEAE), AS BCL-XL/BAK AND MCL-1/BID DUAL INHIBITORS, AND APPROACHES TOWARD THE SYNTHESIS OF KINGIANINS Mohamad Nurul Azmi Mohamad Taib, Yvan Six, Marc Litaudon, Khalijah Awang To cite this version: Mohamad Nurul Azmi Mohamad Taib, Yvan Six, Marc Litaudon, Khalijah Awang. ISOLATION AND IDENTIFICATION OF CYCLIC POLYKETIDES FROM ENDIANDRA KINGIANA GAMBLE (LAURACEAE), AS BCL-XL/BAK AND MCL-1/BID DUAL INHIBITORS, AND APPROACHES TOWARD THE SYNTHESIS OF KINGIANINS . Chemical Sciences. Ecole Doctorale Polytechnique; Laboratoires de Synthase Organique (LSO), 2015. English. tel-01260359 HAL Id: tel-01260359 https://pastel.archives-ouvertes.fr/tel-01260359 Submitted on 22 Jan 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ISOLATION AND IDENTIFICATION OF CYCLIC POLYKETIDES FROM ENDIANDRA KINGIANA GAMBLE (LAURACEAE), AS BCL-XL/BAK AND MCL-1/BID DUAL INHIBITORS, AND APPROACHES TOWARD THE SYNTHESIS OF KINGIANINS MOHAMAD NURUL AZMI BIN MOHAMAD TAIB FACULTY OF SCIENCE UNIVERSITY -

Total Synthesis of Calicheamicin Type Enediyne Natural Products
Total Synthesis of Calicheamicin Type Enediyne Natural Products 17/09/02 Yuki Fujimoto 1 Contents 2 Enediyne Natural Products SSS Me O OMe NHCO 2Me MeO O O H OH HO O N O O O OMe S O OH HO I OH O OMe O NHEt I calicheamicin 1 (calicheamicin type) OMe O O O O CO 2H OH O HN O O OMe OH O O MeH N HO OH O OH O OH dynemicin A (dynemicin type) neocarzinostatin chromophore (chromoprotein type) 3 Bergman Cyclization Jones, R. R.; Bergman, R. G . J. Am. Chem. Soc. 1972 , 94 , 660. 4 Distance of Diyne a) Nicolaou, K. C.; Zuccarello, G.; Ogawa, Y.; Schweiger, E. J.; Kumazawa, T. J. Am. Chem. Soc. 1988 , 110 , 4866. 5 b) Nicolaou, K. C.; Dai, W. M. Angew. Chem. Int. Ed. Engl. 1991 , 30 , 1387. Calicheamicin Type Action Mechanism Nicolaou, K. C.; Dai, W. M. Angew. Chem. Int. Ed. Engl. 1991 , 30 , 1387 6 Contents 7 Introduction of Calicheamicin SSS Me O OMe NHCO 2Me MeO O O H OH HO O N O O O OMe S O OH HO I OH O OMe NHEt I O calicheamicin 1 SSS Me Isolation O 1) NHCO 2Me bacterial strain Micromonospora echinospora ssp calichensis I Total synthesis of calicheamicin 1 HO OH Nicolaou, K. C. (1992, enantiomeric) 2) Danishefsky (1994, enantiomeric) 3) Total synthesis of calicheamicinone Danishefsky, S. J. (1990, racemic) 4) Nicolaou, K. C. (1993, enantiomeric) 5) calicheamicinone Clive, D. L. J. (1996, racemic) 6) (calicheamicin aglycon) Magnus, P. (1998, racemic) 7) 1) Borders, D. -

US5276159.Pdf
||||||||||||| US005276159A United States Patent (19) 11 Patent Number: 5,276,159 Smith et al. 45 Date of Patent: Jan. 4, 1994 (54) DYNEMICIN ANALOGS: SYNTHESES, 56) References Cited METHODS OF PREPARATION AND USE PUBLICATIONS 75 Inventors: Adrian L. Smith, Bishops Stortford, Cabal et al. J.A.C.S. 112-3253 (1990). England; Chan-Kou Hwang, San Schoenen et al. Tetrahedron Lett. 30-3765-3768 Diego, Calif.; Sebastian V. (1989). Wendeborn, La Jolla, Calif.; Kyriacos Kende et al, Tetrahedron Lett. 29-4217-4220 (1988). C. Nicolaou, LaJolla, Calif.; Erwin P. Nicolaou et al. J.A.C.S. 114(23) 8908-21 (1992). Schreiner, Vienna, Austria; Wilhelm Konishi et al., J. Am. Chem. Soc. 112-3715-3716 Stahl, Frankfurt am Main, Fed. Rep. (1990). of Germany; Wei-Min Dai, San Konishi et al. J. Antibiot. 42:1449-1452 (1989). Diego; Peter E. Maligres, La Jolla, Golik et al, J.A.C.S. 109:3461-3462 (1987). both of Calif.; Toshio Suzuki, Golik et al. J.A.C.S. 109:3462-3464 (1987). Niigata, Japan Lee et al J.A.C.S. 109:3464-3466 (1987). Ellestad et al, J.A.C.S. 109:3466-3468 (1987). (73) Assignee: The Scripps Research Institute, La Primary Examiner-Cecilia Tsang Jolla, Calif. Attorney, Agent, or Firm-Dressler, Goldsmith, Shore, (21) Appl. No.: 886,984 Sutker & Milnamow, Ltd. 57) ABSTRACT (22 Filed: May 21, 1992 A fused ring system compound is disclosed that con tains an epoxide group on one side of the fused rings and Related U.S. Application Data an enediyne macrocyclic ring on the other side of the 63 Continuation-in-part of Ser. -

The Chemistry of Nine-Membered Enediyne Natural Products
The Chemistry of Nine-Membered Enediyne Natural Products Zhang Wang MacMillan Group Meeting April 17, 2013 Natural Products Sharing a Unique Structure OH O O H H OH O Me O MeO Cl OH HO O Me Cl O Me O O O H OH OH NC H OH [O] OH R2 cyanosporaside A R sporolide A H 1 [O] R OH [O] O O Cl O Me R1 = H, R2 = Cl Cl MeO H OH or R1 = Cl, R2 = H OH O O HO O O Me O H H Me OH O H OH OH NC OH cyanosporaside B sporolide B Oh, D.-C.; Williams, P. G.; Kauffman, C. A.; Jensen, P. R.; Fenical, W. Org. Lett. 2006, 8, 1021-1024. Buchanan, G. O.; Williams, P. G.; Feling R. H.; Kauffman, C. A.; Jensen, P. R.; Fenical, W. Org. Lett. 2005, 7, 2731-2734. Natural Products Sharing a Unique Structure OH O O H H OH O Me O MeO Cl OH HO O Me Cl O Me O O O H OH OH NC H OH OH cyanosporaside A R sporolide A OH O O + "magic" Cl + "HCl" O Me Cl OH MeO H OH O O HO O O Me O H H Me OH O H OH OH NC OH cyanosporaside B sporolide B Oh, D.-C.; Williams, P. G.; Kauffman, C. A.; Jensen, P. R.; Fenical, W. Org. Lett. 2006, 8, 1021-1024. Buchanan, G. O.; Williams, P. G.; Feling R. H.; Kauffman, C. A.; Jensen, P. R.; Fenical, W. Org. Lett. 2005, 7, 2731-2734. -

(12) United States Patent (10) Patent No.: US 9.260,425 B2 D0 Et Al
USOO9260425B2 (12) United States Patent (10) Patent No.: US 9.260,425 B2 D0 et al. (45) Date of Patent: Feb. 16, 2016 (54) PYRAZOLO3,4-CIPYRIDINE COMPOUNDS WO 2006/042102 A2 4/2006 AND METHODS OF USE WO 2010/022081 A1 2, 2010 WO 2012 O78777 A1 6, 2012 (75) Inventors: Steven Do, San Jose, CA (US); Huiyong Hu, San Mateo, CA (US); Aleksandr OTHER PUBLICATIONS Kolesnikov, San Francisco, CA (US); Dong et al., “QSAR study of Akt protein kinase B (PKB) inhibitors Wendy Lee, San Ramon, CA (US); using support vector machine” Eur J Med Chem. 44(10):4090-7 Vickie H. Tsui, San Francisco, CA (US); (2009). Xiaojing Wang, Foster City, CA (US); Muddassar et al., “Elucidation of binding mode and three dimen Zhaoyang Wen, San Francisco, CA (US) sional quantitative structure-activity relationship studies of a novel series of protein kinase B/Akt inhibitors' J Mol Model. 15(2): 183-92 (73) Assignee: Genetech, Inc., South San Francisco, (Feb. 2009). CA (US) Ohi et al., CAS Registry, Database Accession No. 2003:972059. “Preparation of pyrazole derivatives as JNK inhibitors'. (*) Notice: Subject to any disclaimer, the term of this PCT ISR and Written Opinion of the ISA for PCT/EP2012/065643. patent is extended or adjusted under 35 Wang et al., “Discovery of novel pyrazolo 1.5-alpyrimidines as U.S.C. 154(b) by 0 days. potent pan-Pim inhibitors by structure- and property-based drug design” Bioorg Med Chem Lett. 23(11):3149-53 (Jun. 2013). (21) Appl. No.: 13/571,595 Zhu et al., “Design and synthesis of pyridine-pyrazolopyridine-based inhibitors of protein kinase B/Akt' Bioorg Med Chem. -

Challenges and Opportunities to Develop Enediyne Natural Products As Payloads for Antibody-Drug Conjugates
Antibody Therapeutics, 2021, Vol. 4, No. 1 1–15 doi:10.1093/abt/tbab001 Advance Access Publication on 12 January 2021 Review Article Challenges and opportunities to develop enediyne natural products as payloads for antibody-drug conjugates Ajeeth Adhikari1,2, Ben Shen1,3,4,* and Christoph Rader 2,* 1Department of Chemistry, The Scripps Research Institute, Jupiter, FL 33458, USA, 2Department of Immunology and Microbiology, The Scripps Research Institute, Jupiter, FL 33458, USA, 3Department of Molecular Medicine, The Scripps Research Institute, Jupiter, FL 33458, USA, and 4Natural Products Discovery Center at Scripps Research, The Scripps Research Institute, Jupiter, FL 33458, USA Received: November 23, 2020; Revised: December 23, 2020; Accepted: January 1, 2021 Abstract Calicheamicin, the payload of the antibody-drug-conjugates (ADCs) gemtuzumab ozogamicin (Mylotarg R ) and inotuzumab ozogamicin (Besponsa R ), belongs to the class of enediyne natural products. Since the isolation and structural determination of the neocarzinostatin chromophore in 1985, the enediynes have attracted considerable attention for their value as DNA damaging agents in cancer chemotherapy. Due to their non-discriminatory cytotoxicity towards both cancer and healthy cells, the clinical utilization of enediyne natural products relies on conjugation to an appropriate delivery system, such as an antibody. Here, we review the current landscape of enediynes as payloads of first-generation and next-generation ADCs. STATEMENT OF SIGNIFICANCE: Enediyne natural products -

Docking, Triggering, and Biological Activity of Dynemicin a In
Published on Web 06/11/2005 Docking, Triggering, and Biological Activity of Dynemicin A in DNA: A Computational Study Tell Tuttle,† Elfi Kraka,* and Dieter Cremer Contribution from the Department of Chemistry and Department of Physics, UniVersity of the Pacific, 3601 Pacific AVenue, Stockton, California 95211-0110 Received June 24, 2004; E-mail: [email protected]; [email protected] Abstract: The triggering and biological activity of the naturally occurring enediyne dynemicin A (1) was investigated, both inside and outside the minor groove of the duplex 10-mer B-DNA sequence d(CTACTACTGG)‚d(CCAGTAGTAG), using density functional theory (B3LYP with the 3-21G and 6-31G- (d) basis set), BD(T)/cc-pVDZ (Brueckner doubles with a perturbative treatment of triple excitations), and the ONIOM approach. Enediyne 1 is triggered by NADPH in a strongly exothermic reaction (-88 kcal/ mol), which involves a number of intermediate steps. Untriggered 1 has a high barrier for the Bergman cyclization (52 kcal/mol) that is lowered after triggering to 16.7 kcal/mol due to an epoxide opening and the accompanying strain relief. The Bergman reaction of triggered 1 is slightly exothermic by 2.8 kcal/mol. The singlet biradical formed in this reaction is kinetically stable (activation enthalpies of 19.5 and 21.8 kcal/mol for retro-Bergman reactions) and is as reactive as para-benzyne. The activity-relevant docking mode is an edge-on insertion into the minor groove, whereas the intercalation between base pairs, although leading to larger binding energies, excludes a triggering of 1 and the development of its biological activity. -

Enediyne Natural Products: Biosynthesis and Prospect Towards Engineering Novel Antitumor Agents
Current Medicinal Chemistry, 2003, 10, 2317-2325 2317 Enediyne Natural Products: Biosynthesis and Prospect Towards Engineering Novel Antitumor Agents Ben Shen1,2*, Wen Liu1 and Koichi Nonaka1 1Division of Pharmaceutical Sciences and 2Department of Chemistry, University of Wisconsin, Madison, WI 53705, USA Abstract: This review gives a brief account on the current status of enediyne biosynthesis and the prospective of applying combinatorial biosynthesis methods to the enediyne system for novel analog production. Methods for cloning enediyne biosynthetic gene clusters are first reviewed. A unified paradigm for enediyne biosynthesis, characterized with (a) the enediyne PKS, (b) the enediyne PKS accessory enzymes, and (c) tailoring enzymes, is then presented. Strategies and tools for novel enediyne analog production by combinatorial biosynthesis are finally discussed. The results set the stage to decipher the molecular mechanism for enediyne biosynthesis and lay the foundation to engineer novel enediynes by combinatorial biosynthesis for future endeavors. Keywords: Biosynthesis, C-1027, calicheamicin, combinatorial biosynthesis, enediyne, polyketide synthase. INTRODUCTION categories according to the enediyne core structures. Members of the 9-membered enediyne core sub-category are Neocarzinostatin (NCS), the first member of the enediyne chromoproteins consisting of an apo-protein and the family of antitumor antibiotics, was originally discovered as enediyne chromophore, with N1999A2 (5 ) from a macromolecular antitumor antibiotic from the culture Streptomyces sp. AJ9493 as the only exception that was filtrates of a Streptomyces carzinostaticus strain in 1965 [1]. isolated as a chromophore alone [15]. The apo-protein acts as Although it became clear shortly after its discovery that all a stabilizer and specific carrier for the otherwise unstable biological activities of NCS resided in a nonprotein chromophore and its transport and interaction with target chromophore, the NCS chromophore structure (1) was not DNA. -

WO 2015/112974 Al 30 July 2015 (30.07.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/112974 Al 30 July 2015 (30.07.2015) P O P C T (51) International Patent Classification: (74) Agents: RESNICK, David S. et al; Nixon Peabody LLP, C12Q 1/68 (2006.01) 100 Summer Street, Boston, Massachusetts 021 10 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/US2015/012891 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (22) Date: International Filing BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, 26 January 2015 (26.01 .2015) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, (26) Publication Language: English MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (30) Priority Data: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, 61/93 1,959 27 January 2014 (27.01.2014) US SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicants: THE GENERAL HOSPITAL CORPORA¬ TION [US/US]; 55 Fruit Street, Boston, Massachusetts (84) Designated States (unless otherwise indicated, for every 021 14 (US). -

Etude Des Fonctions Métaboliques De L'oncoprotéine MDM2
Etude des fonctions métaboliques de l’oncoprotéine MDM2 : vers de nouveaux traitements thérapeutiques pour le Liposarcome Madi Cisse To cite this version: Madi Cisse. Etude des fonctions métaboliques de l’oncoprotéine MDM2 : vers de nouveaux traitements thérapeutiques pour le Liposarcome. Médecine humaine et pathologie. Université Montpellier, 2019. Français. NNT : 2019MONTT082. tel-02868641 HAL Id: tel-02868641 https://tel.archives-ouvertes.fr/tel-02868641 Submitted on 15 Jun 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. ! 9/Z8,!65:7!5)9,407!2,!.7(+,!+,!+5*9,:7!! DE L’UNIVERSITÉ DE M (,96,220,7! ! ,I!)D565748!%9INT! ! S?568!@5?N5?968!2+8#!,ABCD! ! :IDNT!@A!LA?EAL?EA!072F!-48,73!)BBGH ! ! Etude des fonctions métaboliques de l’oncoprotéine !"!8$6$3%/0$$%$+,23%.24$1/.11%2%+10$134/.5%211.2%0$ 5,2/$7%$ 15,0./9,2%7 $ $ 6LTMAINTA!J9?!39>4!24MMT! .A!BD!+T?AHLLA!@MBG! ! %5NI!69!>4LA?N453!>8!.9ON4N49!.-4(7,8! ! !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!18P9IN!68!ENLR!?5HK5IT!@A! ! 3 !GA!1?!39??!809)5,! 6LTMD@AIN!@N!ENLR! 3HA !.8!1?!.9ONDN49!.04(7,8! 14?A?NLD?A!@A!NEYIA! 3HA !68!1?!85JEDA!T088,:7! *9JJ5LN8NL! 3 !GA!1?!-L93?F!9(.,1(! *9JJ5LN8NL! 3 !GA!1?!-LT@TLD?!2#0)(,! ,Z9HDI9:8NL! 3 !GA!6L!1A93X2E9?68I!65790-8! ,Z9HDI9:8NL! 3 !GA!1?!.9NLAIN!.,!20F! Chef d’équipe ! ! ! ! Remerciements Les personnes qui me connaissent bien diront que je suis un optimiste et rarement stressé.