Food Chemistry 215 (2017) 7–16
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Telstar Satellite Rocket Launched
Today ' 3 i' ^^^^ ^ 21,950 lafad. Lej» tod|bt In the 4k, 9M' Weratr, page 2.' DIAL SH 1-0010 VOL. '85 NO 224. IMIM* itltr. MoniStT thronm frliUr. B^oond oitu Pwttf* rvu °3' «'-'• •"* raid at KM Buk and at ASditiooU XUllnc OtOei. RED BANK, N. J., TUESDAY, MAY 7, 1963 7c PER COPY PAGE ONE Senate to Probe Race) Tracks' Expenses TRENTON (AP)-The ttate sen- Hillery, R-Merris and Wayne Du- bill and that was why he support- the committee membership and Sandman on what basis the com- ate has deckled to do tome of moot, It-Warren, sponsored a ed Stamler's. < warned jokingly that any commit- mittee members were chosen. tee member caught with white- its own Investigating into a $2.4 measure calling for a similar In- "What he meant by not enough He said no committee member million expense account submit- vestigation, but their resolution votes for my bill is that he wash on his fingers would be bounced from the committee. could be running for re-election ted by two race tracks lor operat- did not include Cowgili and was couldn't get six votes in the GOP and have a race track in his caucus.. .there were 10 Demo- He-named Dumont as chairman ing a special 30-day season last limited to a senate committee of county. year to provide money for shore five, crats ready to vote for it here," and appointed Sens. Hillery; Stam- relief. Gives Reason Cowgili said. ler; waiiam F. Kelly, D-Hud- The only senator who,is run- The senate, after a heated ex- Sandman said on the senate floor As soon as the vote was tak- son, and John A. -
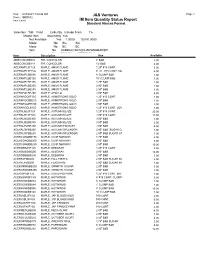
J&S Ventures IM Item Quantity Status Report
Run : 9/27/2021 7:20:46 AM J&S Ventures Page 1 Form : IMR0032 Item Level IM Item Quantity Status Report Standard Abecas Format Selection : Tab Field Order By Include From To Master Item Ascending Yes Net Available Yes 1.0000 10,000.0000 Mode No BG BG Mode No BC BC Item No HARBALCAR100 HARWEEBARSPI Item No ZZAVAIL ZZTRESTAK Item Description Size Available ABIECONCBB072 FIR, CONCOLOR 6' B&B 2.00 ABIECONCBB144 FIR, CONCOLOR 12' B&B 2.00 ACERAMFL0F125 MAPLE, AMUR FLAME 1.25" #15 CONT. 5.00 ACERAMFL0F15G MAPLE, AMUR FLAME 6' CL. #15 CONT. MULTI STEM 4.00 ACERAMFLBB096 MAPLE, AMUR FLAME 8' CLUMP B&B 1.00 ACERAMFLBB120 MAPLE, AMUR FLAME 10' CLUMP B&B 1.00 ACERAMFLBB175 MAPLE, AMUR FLAME 1.75" B&B 1.00 ACERAMFLBB200 MAPLE, AMUR FLAME 2.00" B&B 1.00 ACERAMFLBB250 MAPLE, AMUR FLAME 2.50" B&B 1.00 ACERAPOLBB200 MAPLE, APOLLO 2.00" B&B 5.00 ACERARGO0F125 MAPLE, ARMSTRONG GOLD 1.25" #15 CONT. 1.00 ACERARGOBB200 MAPLE, ARMSTRONG GOLD 2.00" B&B 1.00 ACERARGOBB250 MAPLE, ARMSTRONG GOLD 2.50" B&B 2.00 ACERARGOLB125 MAPLE, ARMSTRONG GOLD 1.25" #15 CONT. LOW BRANCH 1.00 ACERAUBL0F125 MAPLE, AUTUMN BLAZE 1.25" #15 CONT. 20.00 ACERAUBL0F20G MAPLE, AUTUMN BLAZE 2.00" #20 CONT. 17.00 ACERAUBLBB300 MAPLE, AUTUMN BLAZE 3.00" B&B 1.00 ACERAUBLBB350 MAPLE, AUTUMN BLAZE 3.50" B&B 2.00 ACERAUFABB200 MAPLE, AUTUMN FANTASY 2.00" B&B 4.00 ACERAUSPBB200 MAPLE, AUTUMN SPLENDOR 2.00" B&B SUGAR CADDO 1.00 ACERAUSPBB250 MAPLE, AUTUMN SPLENDOR 2.50" B&B SUGAR CADDO 1.00 ACERCONOBB175 MAPLE, CLNR NORWAY 1.75" B&B 2.00 ACERCONOBB200 MAPLE, CLNR NORWAY 2.00" B&B 5.00 ACERCONOBB250 MAPLE, CLNR NORWAY 2.50" B&B 19.00 ACERDEBO0F125 MAPLE, DEBORAH 1.25" #15 CONT. -

Modificaciones En Factores Relacionados Con El Aroma Y La Textura De La Manzana, Melocotón Y Nectarina Durante La Maduración Y La Post- Cosecha
Modificaciones en factores relacionados con el aroma y la textura de la manzana, melocotón y nectarina durante la maduración y la post- cosecha Abel Ortiz Catalán R " Q $% R &' ' ()$ + , -.)/ " 1 2 - 234 $61Q&' ' ( )$ + ,R$ 6 $ Q $ % R 1 $ 2 ( , ) 1 + -7 8 9 8 92 - ) , ' 2 -.)/ 2 - 234 $&:;<< " " " # " "%& & " ' " % " % % ( '" * % & ! # # ! ! $ + , % & % & '! $ ! ( # $ ! $ & " ( &" -./ ) ' * !! 9 ! $Q, - R / 0, 12 3 4 5 $6/-73%(8 ' & ! 9) $Q:!;<=!>R / 0, 12 3 *1 9)9 ' $ ! ! %) ! $Q-2 R / 04 ,2 2, 12 3 ' ' / ! $ Q:!; ) <=!>R / 04 ,, 12 3 *112 9))> ' / 7 $ $ ! #! # >) Q, - R $ # ! / 04 ,, 12 3 *11 9 ' / / '! $Q- 2 R $ ! $ 0 ( 7 $ / 04 ,, 12 3 *112 9(9) ' / ? ! $ # $ ! ! # Q- 2 R / 04 ,, 12 22 3 ' ' / * Q7 # R $ ! & / 0, 12 24 ,2 3 *11 %)>( ' 3 @ $ ! ! $Q, - R / 04 ,, 12 3 *11 >((% ' 3 & ! # @ $ @ # $Q:!;<=!>R / 0, 12 3 *1 A ((%B; #((( ' ' 3 @ #! $ $ % Q? -

Some Fruit and Descriptions, with Apple Photos Apples, Pears, Plums
1 Some Fruit and Descriptions, with Apple Photos Apples, Pears, Plums, Prunes, Zwetschen (Zwetschgen), d’Agen, Damson A Note About the Tables: A = Roadside seedling; collected and named by Katrina Richards. B = NZ, 2005. Open pollinated apple seedling raised by Katrina Richards. C = NZ, 20th Century. A seedling found by chance on Richards’ Orchard. D = For various reasons, the variety needed a name, so we gave it one. We have made all efforts to ensure our scion wood & information is correct. Unless otherwise attributed, photos are by Katrina Richards, who holds copyright for these images. APPLES Adam’s UK, 1826. Orange-red blush and stripes, some Pearmain russet, long shape, medium sized fruit. Nutty, aromatic, firm, juicy. Mid-season. Akane Japan, Bright red skin, snow white flesh, 1937. flattish. Sweet, juicy, crisp/firm. Eat, cook, juice, cider. Pick mid-February until May. Disease resistant. Alexander Ukraine, Green with red stripes, large fruit. 1700s. Cook, can also eat. Mid-season. From SCES collection. Image: wikicommons www.nationalfruitcollection.org.uk/ Altländer Germany, Red with broken stripes. Fruity, tart. Pfannkuchen- 1840. Cooks to tasty soft puree. Use in apfel apple pancakes. Mid-season. Aria B Block red with red stripes. Sweet, juicy, crisp, hint of honey. Black Spot resistant? Ready in February. 2 Awatere A Stripes, large fruit, heavy crops. Excellent cooker. Suffers from bitter pit (calcium disorder) on Moutere Clay. Ballarat Australia, Round, large, pale green, pink blush. 1870s. Tart, juicy. Cooks to puree. Late season. Excellent keeper. May keep 6 months without refrigeration. Beauty of Bath UK, 1864. Red stripes and attractive spots, flat. -

Yale French Studies Structuralism
Yale French Studies Structuralism Double Issue - Two Dollars Per Copy - All ArticlesIn English This content downloaded from 138.38.44.95 on Sun, 03 Jan 2016 19:52:13 UTC All use subject to JSTOR Terms and Conditions Yale French Studies THIRTY-SIX AND THIRTY-SEVEN STRUCTURALISM 5 Introduction The Editor LINGUISTICS 10 Structureand Language A ndreMartinet 19 Merleau-Pontyand thephenomenology of language PhilipE. Lewis ANTHROPOLOGY 41 Overtureto le Cru et le cuit Claude Levi-Strauss 66 Structuralismin anthropology Harold W. Scheffler ART 89 Some remarkson structuralanalysis in art and architecture SheldonNodelman PSYCHIATRY 104 JacquesLacan and thestructure of the unconscious JanMiel 112 The insistenceof the letterin the unconscious JacquesLacan LITERATURE 148 Structuralism:the Anglo-Americanadventure GeoffreyHartman 169 Structuresof exchangein Cinna JacquesEhrmann 200 Describingpoetic structures: Two approaches to Baudelaire'sles Chats Michael Riffaterre 243 Towards an anthropologyof literature VictoriaL. Rippere This content downloaded from 138.38.44.95 on Sun, 03 Jan 2016 19:52:13 UTC All use subject to JSTOR Terms and Conditions BIBLIOGRAPHIES 252 Linguistics ElizabethBarber 256 Anthropology Allen R. Maxwell 263 JacquesLacan AnthonyG. Wilden 269 Structuralismand literarycriticism T. Todorov 270 Selectedgeneral bibliography The Editor Cover: "Graph,"photograph by JacquesEhrmann. Editor, this issue: Jacques Ehrmann; Business Manager: Bruce M. Wermuth; General Editor: Joseph H. McMahon; Assistant: Jonathan B. Talbot; Advisory Board: Henri Peyre (Chairman), Michel Beaujour, Victor Brombert, Kenneth Cornell, Georges May, Charles Porter Subscriptions:$3.50 for two years (four issues), $2.00 for one year (two issues), $1.00 per number.323 W. L. HarknessHall, Yale University,New Haven, Connecticut.Printed for YFS by EasternPress, New Haven. -

Plant Availability
Plant Availability Product is flying out the gates! Availability is current as of 4/11/20 and is subject to change without notice. Call us to place an order for pick up or discuss details about curbside, local delivery for the Clovis/Fresno area. 559-255-6645 Or visit us! Our outdoor nursery is located on 10 acres at 7730 East Belmont Ave Fresno, CA. 93737 Availability in alphabetical order by botanical name. Common Name Botanical Name Size Loc. Avail Retail Glossy Abelia Abelia G Compacta Variegata * #5 R280A 15 $ 24.99 Confetti Abelia Abelia G Confettii #5 RETAIL 7 $ 28.99 Glossy Abelia Edward Goucher Abelia G Edward Goucher * #5 R280A 11 $ 19.99 'Kaleidoscope' Abelia Abelia Kaleidoscope Pp#16988 * #3 RET 1 $ 29.99 Passion Chinese Lantern Abutilon Patio Lantern Passion 12 cm R101 170 $ 7.99 Bear's Breech Acanthus mollis #5 R340B 30 $ 23.99 Trident Maple Acer Buergerianum #5 R424 3 $ 36.99 Miyasama Kaede Trident Maple Acer Buergerianum Miyasama Kaede #15 R520B 1 $ 159.99 Trident Maple Acer Buergerianum Trident #15 R498 3 $ 89.99 Trident Maple Acer Buergerianum Trident #15 R442 5 $ 89.99 Autumn Blaze Maple Acer Freemanni Autumn Blaze 24 box R800 2 $ 279.00 Autumn Blaze Maple Acer Freemanni Autumn Blaze #15 R442 3 $ 84.99 Autumn Blaze Maple Acer Freemanni Autumn Blaze 30 box R700 4 $ 499.00 Autumn Blaze Maple Acer Freemanni Autumn Blaze #5 R425 19 $ 39.99 Autumn Fantasy Maple Acer Freemanni Autumn Fantasy #15 R440 6 $ 84.99 Ruby Slippers Amur Maple Acer G Ruby Slippers 24 box R700 12 $ 279.00 Flame Maple Multi Acer Ginnala Flame Multi 30 box R700 2 $ 499.00 Flame Amur Maple Acer Ginnala Flame Std. -

R Graphics Output
Aberystwyth University Development of a minimal KASP marker panel for distinguishing genotypes in apple collections Winfield, Mark; Burridge, Amanda; Ordidge, Matthew; Harper, Helen; Wilkinson, Paul; Thorogood, Danny; Copas, Liz; Edwards, Keith; Barker, Gary Published in: PLoS One DOI: 10.1371/journal.pone.0242940 Publication date: 2020 Citation for published version (APA): Winfield, M., Burridge, A., Ordidge, M., Harper, H., Wilkinson, P., Thorogood, D., Copas, L., Edwards, K., & Barker, G. (2020). Development of a minimal KASP marker panel for distinguishing genotypes in apple collections. PLoS One, 15(11), [e0242940]. https://doi.org/10.1371/journal.pone.0242940 Document License CC BY General rights Copyright and moral rights for the publications made accessible in the Aberystwyth Research Portal (the Institutional Repository) are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the Aberystwyth Research Portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the Aberystwyth Research Portal Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. -

Telstar Triumphs in Space Mission
Distributior Today ** WDBANK * (8,725 tamemir with hlfk Mar N. 1 *>;' 4ajr fair and pleasant Sea Weather, paje 2. Dial SH I-00I0 VOL. 85, NO. 11 J tally, radij. Uoood Ciu» PMOI> RED BANK, N. J., WEDNESDAY, JULY 11, 1962 ItU at R*4 uutteui IUIUHI otutM. 7c PER COPV PACE ONE Makes $4 Million Phone Call By RUSSELL P. RAUCH Thus, my voice had to be carried over regular circuits HOLMDEL-I made a W million telephone call last night. to Andover before being beamed to Telstar. Telstar Triumphs The conversation was short — but it was still unique. Telstar did its job — received, amplified and transmitted The sound of my voice and that of the party on the other end of the line had to travel 3,500 miles into space and back my voice back down to Andover and Mrs. Penley. again. Yet the distance between us was only 350 miles. WEATHER QUESTIONS At the other end of the line a woman answered. The conversation went like this: She'was Mrs. Nelle Penley, a staff reporter for the Bangor Mrs. Penley: Hello, Is this Russ Rauch? Daily News. She was sitting in a tracking station in Andover, Mr. Rauch: Yes it is. How are you? Maine. In Space Mission Mrs. Penley: Just fine. This reporter was one of four newsmen at the tracking Mr. Rauch: How is the weather up there in Maine? station here on Crawford's Hill selected to talk to a like num- Mrs. Penley: Cool. NEW YORK (AP)-Telstar, by the Bell Telephone Lab- Ultimately, the instantaneous, signals beamed to it. -
Brkini Fruit Road
Brkini Fruit Road Brkini, November 2011 TABLE OF CONTENTS 1 FRUIT-GROWING DISTRICT OF BRKINI 2 FRUIT GROWING IN BRKINi – tHE PAST 3 ... AND THE PRESENT ............... 4 FRUIT FOR HEALTH AND WELLBEING 5 RECIPES FOR SWEET TOOTH SATISFACTION 6 A PRESENTATION OF FARMS 7 SIGHTS 8 EXCURSIONS 9 MAP “ONE PLUM, ONE GLASS”, … say the men sitting next to copper stills in the winter cooking the Brkinski Slivovec plum brandy. Brkini ... a rolling landscape of many faces. Playful in the spring, friendly and leisurely in the summer, dreamy in the autumn fogs and strangely silent in the winter. All its hues seem a bit distanced from the contemporary world where we constantly rush and yearn for something ... what is it that we’re longing for? Somewhere along the way between nucle- ated villages, the time appears to slow down and some previ- ously highly important things become a little less important. As you stand on the top of a hill, looking at a buzzard silently drifting into infinity, the tranquillity and softness of the land- scape take you over. Welcome to this distinct world, among the people living here for centuries. Lone and hospitable, cheerful and melanchol- ic, playfully roguish and wise, and sometimes hidden behind a wall of seeming toughness. Just like the place where they live. 1 FRUIT-GROWING DISTRICT OF BRKINI Brkini are famous for producing quality fruit, notably apples and plums, and for the Brkinski Slivovec plum brandy. Brkini are a hilly region located in four municipalities: Divača, Hrpelje-Kozina, Ilirska Bistrica and Pivka. The fruit-growing dis- trict of Brkini includes not only the hills but also the Reka River valley, the Vreme Valley and the Košana Valley to the northeast, the Divača and Kozina Karst to the northwest and southwest, respectively, and Čičarija. -

Innovation Is in Ourdna Letter from Our Contents Page 12 Our Editor Features
Magazine for the Science & Technology Innovation Center in Middletown, NJ • Premier Edition Honoring the Past, Creating the Future Innovation is in ourDNA Letter from Our Contents page 12 our Editor Features 2 The History of AT&T 94 Data Transmssion — Fax Welcome to our Science & Technology Innovation Center of Middletown, New Jersey magazine premier edition. The new Innovation Center is a place 12 The Transistor 100 Cellular Phones of inspiration and learning from the history of AT&T and significant inventions that our company has created over the past 142+ years that contribute to 13 Bell Solar Cell 104 Project AirGig™ the advancement of humanity. Over my years at AT&T, I have spoken to many The Telstar Project Our Contributors people who never knew that AT&T had a history of innovation in so many 16 109 areas beyond the creation of the telephone by Alexander Graham Bell. Coax Cable 24 Throughout the years, AT&T has been a key player in local and long-distance 30 Fiber Optics in the AT&T Network voice telephony, motion pictures, computers, the cable industry, wireless, and Science & Technology broadband. AT&T has served the nation’s telecommunication needs and par- 34 Vitaphone and Western Electric ticipated in many technology partnerships in every industry throughout the globe. The breadth of technology and innovation goes on and on, but a 44 Picturephone Irwin Gerszberg few of the innovations you might see at our new Innovation Center include: Innovation ground-to-air radio telephony, motion picture sound, the Telstar satellite, Theseus Honoring the Past, Creating the Future AVP Advanced Technology Research 48 telephone switching, the facsimile machine, military radar systems, the AT&T Science & Technology transistor, undersea cable, fiber communications, Picturephone via T1’s, coin 50 A Short History of UNIX™ EDITOR-IN-CHIEF Innovation Center phones, touch-tone dialing, AMPS cellular phones, UNIX™ and C language Irwin Gerszberg programming. -

DS Govt Cov Idaho2003
2003 AND SUCCEEDING CROP YEARS FEDERAL CROP INSURANCE CORPORATION ELIGIBLE PLANT LIST AND PLANT PRICE SCHEDULE NURSERY CROP INSURANCE PROGRAM • IDAHO • OREGON • WASHINGTON The price for each plant and size listed in the Eligible Plant List and Plant Price Schedule is your lowest wholesale price, as determined from your wholesale catalogs or price lists submitted in accordance with the Special Provisions, not to exceed the maximum price limits included in this Schedule. Insurable plants damaged prior to the attachment of insurance coverage will be insured at a reduced value until such plants have fully recovered from damage. CONTENTS INTRODUCTION Crop Insurance Nomenclature Format Determining Eligible Plant List Price of Unlisted Cultivars Crop Type and Optional Units Storage Keys Hardiness Zone Designations Container Insurable Hardiness Zones Field Grown Minimum Hardiness Zones Plant Size SOFTWARE AVAILABILITY System Requirements Sample Report INSURANCE PRICE CALCULATION Examples of Price Calculation Crop Type Base Price Tables ELIGIBLE PLANT LIST AND PLANT PRICE SCHEDULE APPENDIX A County Hardiness Zones B Storage Keys C Insurance Price Calculation Worksheet D Container Volume Calculation Worksheet E FCIC Container Definitions The DataScape Guide to Commercial Nomenclature is used in this document by the Federal Crop Insurance Corporation (FCIC), an agency of the United States Department of Agriculture (USDA), with permission. Permission is given to use or reproduce this Eligible Plant List and Plant Price Schedule for purposes of administering the Federal Crop Insurance Corporation’s Nursery Insurance program only. The DataScape Guide to Commercial Nomenclature is published in electronic format by DataScape, LLC, 1250 South Grove Avenue, Suite 302, Barrington, IL 60010. -

2016 Week 17 PREMIER COLOR AVAILABILITY.Xlsx
Customer: (760)XX CURRENT AVAILABILITY AS OF: po# CITY: 723-7776 w e e k 1 7 FLAT# INV# LIST# Monday, April 25, 2016 ORDER CUT-OFF: 12 noon the day prior for delivery to you area. Out of state order cut-off varies, please inquire FAX #: MARK'S 760-723-7772 ANDY'S 760-723-9933 ERIC'S 760-723-8805 8 " S E L E C T 4" L I M I T E D 4" A N N U A L , continued MASTERPIECE COMBOS 4 POTS/PER FLAT BLACK round pot - 16 count COLEUS 16" Terra Cotta Clay pots SCAEVOLA CYPERUS superfine rainbow mix full plants top model blue bud/bloom cleopatra full plants CRAESPEDIA chef's reward full plants STACHYS king tut full plants drumstick full plants citrus bud/bloom bella grigio full plants SENETTI perciallis DAHLBERG DAISY fiesta bud/bloom deep blue bud/bloom golden fleece bud/bloom full moon bud/bloom 8 " E D I B L E DAHLIA julie's jewels bud/bloom 4 POTS/PER FLAT 4 " 25 count decorative giants mix full plants midnight bud/bloom ARTICHOKE green square pot - 25 count DIANTHUS outREDgeous bud/bloom emerald full plants PETUNIA grandiflora telstar burgundy bud/bloom pretty in pink bud/bloom opera full plants limbo burgundy bud/bloom telstar carmine rose bud/bloom limbo mix bud/bloom telstar crimcon bud/bloom 12" S E L E C T 1 G S H O W C A S E telstar mix bud/bloom round cache pot EACHES square black pot 9 PER FLAT 4 " 25 count F R U I T telstar pink bud/bloom ARGYRANTHEMUM ANIGOZANTHUS green square pot - 25 count telstar purple bud/bloom butterfly yellow bud/bloom red bud/bloom STRAWBERRY telstar salmon bud/bloom ARTICHOKE GERANIUM pretty in pink bud/bloom