Synthesis of New Chiral Diaryliodonium Salts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Automated Flow Synthesis of Fluorine Containing Organic Compounds
The automated flow synthesis of fluorine containing organic compounds by CHANTAL SCHOLTZ Submitted in partial fulfilment of the requirements for the degree PHILOSOPHAE DOCTOR In the Faculty of Natural & Agricultural Sciences UNIVERSITY OF PRETORIA PRETORIA Supervisor: Dr D.L. Riley February 2019 DECLARATION I, Chantal Scholtz declare that the thesis/dissertation, which I hereby submit for the degree PhD Chemistry at the University of Pretoria, is my own work and has not previously been submitted by me for a degree at this or any other tertiary institution. Signature :.......................................... Date :..................................... ii ACKNOWLEDGEMENTS I would herewith sincerely like to show my gratitude to the following individuals for their help, guidance and assistance throughout the duration of this project: My supervisor, Doctor Darren Riley, for his knowledge and commitment. Thank you for being a fantastic supervisor and allowing me the opportunity to learn so many valuable skills. My husband, Clinton, for all your love, support and patience and for always being there for me. You are the best. My family, for all the encouragement and support you gave me as well as always believing in me. I will always appreciate what you have done for me. Mr Drikus van der Westhuizen and Mr Johan Postma for their assistance at the Pelchem laboratories with product isolation and characterisation. Dr Mamoalosi Selepe for NMR spectroscopy services, Jeanette Strydom for XRF services and Gerda Ehlers at the UP library for her invaluable assistance. All my friends and colleagues for the continuous moral support, numerous helpful discussions and necessary coffee breaks. My colleagues at Chemical Process Technologies for their ongoing support and motivation, especially Dr Hannes Malan and Prof. -

Reduction of Aldehydes and Ketones to Their Corresponding Alcohols in 1- Butyl-3-Methylimidazolium Tetrafluoroborate
REDUCTION OF ALDEHYDES AND KETONES TO THEIR CORRESPONDING ALCOHOLS IN 1- BUTYL-3-METHYLIMIDAZOLIUM TETRAFLUOROBORATE Josiane Ayingeneye Dissertation submitted in fulfilment of the academic requirements for the degree of Master of Science in the School of Chemistry and Physics, University of KwaZulu-Natal, Durban. Supervisor: Prof Vincent O. Nyamori February 2018 Abstract The use and release of volatile organic solvents (VOS) to the atmosphere have become detrimental to both human health and his environment. In addition, the non-recyclability and the excessive consumption of these solvents in chemical industry influence their high costs. This has incited the growing need towards the design of new processes and benign solvents, helping in minimizing these raised issues. Ionic liquids (ILs) which are acknowledged as green and environmentally friendly solvents, are assigned as promising alternatives for replacing these VOS. The review of some ILs aspects such as structure, synthesis methods, physicochemical properties, solvent applications in reduction reactions, and their recyclability gave an impressive trend and a motivation towards our work. Synthesis of 1-butyl-3- methylimidazolium tetrafluoroborate ([BMIM][BF4]), as typical IL and investigation of its solvent efficiency, in the reduction of aldehydes and ketones to their corresponding alcohols, were the core objectives of this research. [BMIM][BF4] was synthesized via microwave (MW) and conventional methods. The characterization of the synthesized [BMIM][BF4] was done by means of Fourrier transform infrared (FTIR), nuclear magnetic resonance (1H- and 13C-NMR,) and liquid chromatograph- mass spectroscopy (LC-MS) techniques. Physicochemical properties of [BMIM][BF4] related to its solvent application, such as water content, density, viscosity and thermal stability, were explored. -

Durham E-Theses
Durham E-Theses Selective Fluorination Strategies BREEN, JESSICA,RUTH How to cite: BREEN, JESSICA,RUTH (2012) Selective Fluorination Strategies, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/3479/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk Abstract There is a great interest in the synthesis of fluorinated aromatic and heterocyclic compounds, which have a range of applications in the pharmaceutical industry. Many common routes to these compounds, however, are low yielding and or/expensive. This thesis is concerned with novel methods for the synthesis of fluoro-aromatics and fluoro- pyrazoles using conventional fluorinating agents, such as Selectfluor™, as well as using elemental fluorine and the flow reactor technology developed in Durham. Firstly, elemental fluorine was used to fluorinate a range of aromatics containing electron-donating substituents, using both batch and flow methods. -

Download Date 07/10/2021 01:33:42
Functional ionic liquids in crystal engineering and drug delivery Item Type Thesis Authors Bansode, Ratnadeep V. Rights <a rel="license" href="http://creativecommons.org/licenses/ by-nc-nd/3.0/"><img alt="Creative Commons License" style="border-width:0" src="http://i.creativecommons.org/l/by- nc-nd/3.0/88x31.png" /></a><br />The University of Bradford theses are licenced under a <a rel="license" href="http:// creativecommons.org/licenses/by-nc-nd/3.0/">Creative Commons Licence</a>. Download date 07/10/2021 01:33:42 Link to Item http://hdl.handle.net/10454/14563 University of Bradford eThesis This thesis is hosted in Bradford Scholars – The University of Bradford Open Access repository. Visit the repository for full metadata or to contact the repository team © University of Bradford. This work is licenced for reuse under a Creative Commons Licence. FUNCTIONAL IONIC LIQUIDS FOR USE IN CRYSTAL ENGINEERING AND DRUG DELIVERY Ratnadeep Vitthal BANSODE Submitted for the Degree of Doctor of Philosophy School of Life Sciences University of Bradford 2016 Abstract Ratnadeep Vitthal Bansode Functional ionic liquids in crystal engineering and drug delivery Key words: Phosphonium ionic liquids, imidazolium ionic liquids, synthesis, pharmaceutical drugs, crystallisation, solubility, pharmaceuticals, API-ILs synthesis and drug release. The objective of this research is to explore the use of ionic liquds in crystal engineering and drug delivery. Ionic liquids have a wide range of applications in pharmaceutical field due to their unique physicochemical propertie ssuch as chemical, thermal stability, low melting point, nonvolatility, nonflamability, low toxicity and recyclability which offer unique and interesting potential for pharmaceuitcal applications. -

Sodium Tetrafluoroborate
Sodium tetrafluoroborate sc-253597 Material Safety Data Sheet Hazard Alert Code EXTREME HIGH MODERATE LOW Key: Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Sodium tetrafluoroborate STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY0 HEALTH3 HAZARD INSTABILITY0 SUPPLIER Santa Cruz Biotechnology, Inc. 2145 Delaware Avenue Santa Cruz, California 95060 800.457.3801 or 831.457.3800 EMERGENCY ChemWatch Within the US & Canada: 877-715-9305 Outside the US & Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 SYNONYMS NaBF4, "sodium fluoroborate", "sodium borofluoride", "sodium tetrafluoroborate", "borate (1-), tetrafluoro-, sodium", "sodium tetrafluoro borate" Section 2 - HAZARDS IDENTIFICATION CHEMWATCH HAZARD RATINGS Min Max Flammability: 0 Toxicity: 3 Body Contact: 4 Min/Nil=0 Low=1 Reactivity: 0 Moderate=2 High=3 Chronic: 2 Extreme=4 CANADIAN WHMIS SYMBOLS 1 of 9 EMERGENCY OVERVIEW RISK Risk of serious damage to eyes. Irritating to respiratory system and skin. Skin contact may produce health damage*. Cumulative effects may result following exposure*. Inhalation and/or ingestion may produce serious health damage*. * (limited evidence). POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED ■ The material can produce severe chemical burns within the oral cavity and gastrointestinal tract following ingestion. ■ Accidental ingestion of the material may be seriously damaging to the health of the individual; animal experiments indicate that ingestion of less than 40 gram may be fatal. ■ Fluoride causes severe loss of calcium in the blood, with symptoms appearing several hours later including painful and rigid muscle contractions of the limbs. Cardiovascular collapse can occur and may cause death with increased heart rate and other heart rhythm irregularities. -

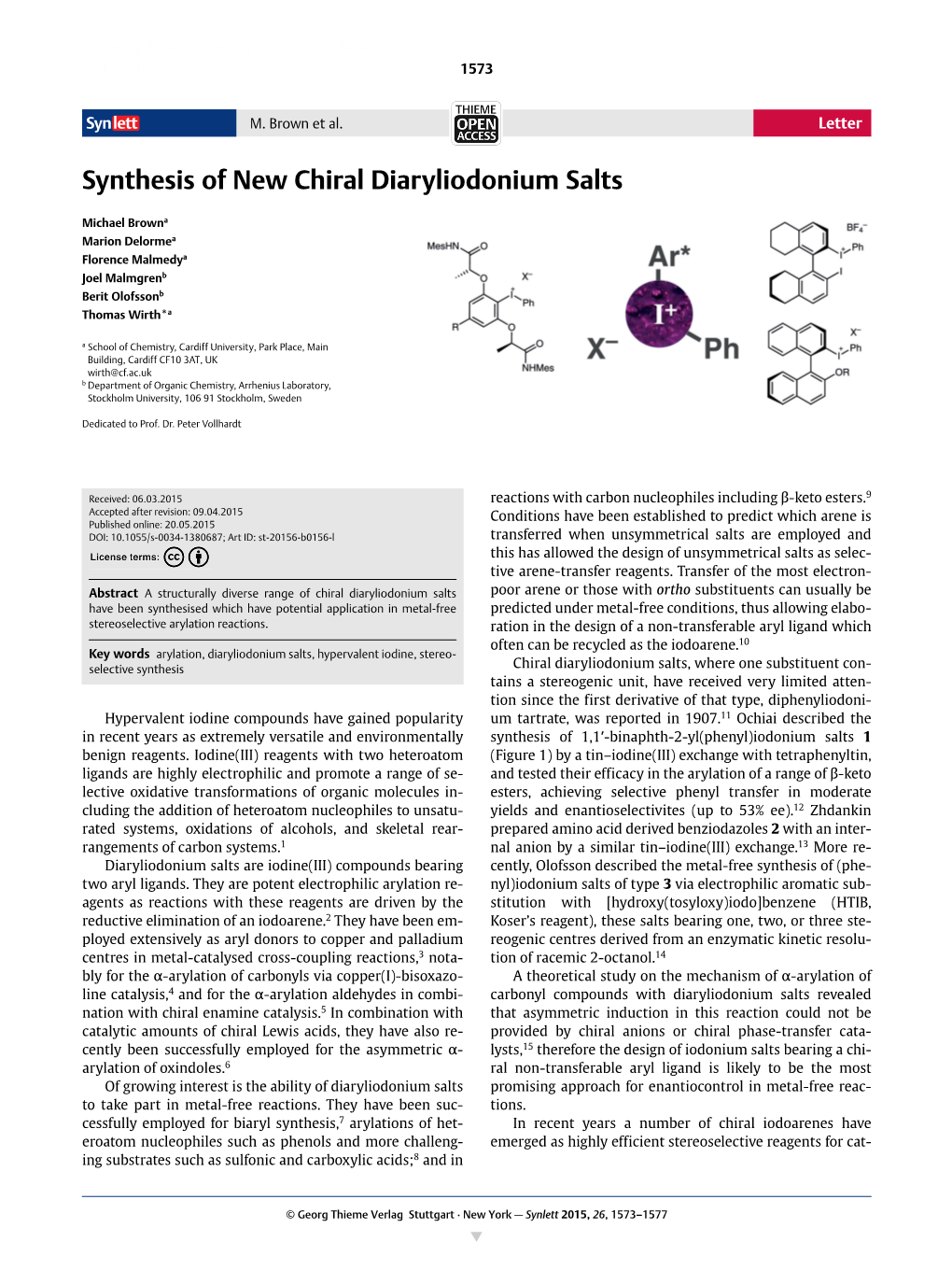
Synthesis of New Chiral Diaryliodonium Salts
SYNLETT0936-52141437-2096 © Georg Thieme Verlag Stuttgart · New York 2015, 26, 1573–1577 1573 letter Syn lett M. Brown et al. Letter Synthesis of New Chiral Diaryliodonium Salts Michael Browna Marion Delormea Florence Malmedya Joel Malmgrenb Berit Olofssonb Thomas Wirth*a a School of Chemistry, Cardiff University, Park Place, Main Building, Cardiff CF10 3AT, UK [email protected] b Department of Organic Chemistry, Arrhenius Laboratory, Stockholm University, 106 91 Stockholm, Sweden Dedicated to Prof. Dr. Peter Vollhardt Received: 06.03.2015 reactions with carbon nucleophiles including β-keto esters.9 Accepted after revision: 09.04.2015 Conditions have been established to predict which arene is Published online: 20.05.2015 DOI: 10.1055/s-0034-1380687; Art ID: st-20156-b0156-l transferred when unsymmetrical salts are employed and this has allowed the design of unsymmetrical salts as selec- tive arene-transfer reagents. Transfer of the most electron- Abstract A structurally diverse range of chiral diaryliodonium salts poor arene or those with ortho substituents can usually be have been synthesised which have potential application in metal-free predicted under metal-free conditions, thus allowing elabo- stereoselective arylation reactions. ration in the design of a non-transferable aryl ligand which often can be recycled as the iodoarene.10 Key words arylation, diaryliodonium salts, hypervalent iodine, stereo- selective synthesis Chiral diaryliodonium salts, where one substituent con- tains a stereogenic unit, have received very limited atten- tion since the first derivative of that type, diphenyliodoni- Hypervalent iodine compounds have gained popularity um tartrate, was reported in 1907.11 Ochiai described the in recent years as extremely versatile and environmentally synthesis of 1,1′-binaphth-2-yl(phenyl)iodonium salts 1 benign reagents. -

Jenna Raunio Base-Catalysed Condensation of Aryl Aldehydes and Valine-Derived Boroxazolidones
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Trepo - Institutional Repository of Tampere University JENNA RAUNIO BASE-CATALYSED CONDENSATION OF ARYL ALDEHYDES AND VALINE-DERIVED BOROXAZOLIDONES Master of Science Thesis Examiners: Professor Robert Franzén Academy Research Fellow Nuno R. Candeias Examiners and topic approved by the Faculty Council of the Faculty of Engineering Sciences on 8th of June 2016 II ABSTRACT RAUNIO, JENNA: Base-catalysed condensation of aryl aldehydes and valine- derived boroxazolidones Tampere University of Technology Master of Science Thesis, 50 pages, 66 Appendix pages October 2016 Master’s Degree Programme in Science and Engineering Major: Materials Chemistry Examiners: Professor Robert Franzén, Academy Research Fellow Nuno R. Candeias Keywords: imine condensation, organoboron compounds, N-B bond, boroxazol- idones, aryl aldehydes, base catalysis Imines are an important group of compounds for many chemical reactions in organic chemistry, mostly as electrophiles. In nature, imines are important for the transamina- tion reaction. N-B bonds are interesting because they can be thought of as an analogy to a C-C bond. However, unlike a C-C bond, the N-B bond is polarized. Imines and N-B bond-containing compounds both have similar potentials as pharmaceuticals. Both of these groups can have antibacterial, antifungal and anticancer effects. The N-B bond focused on in this thesis is formed when an amino acid reacts with a boron compound to form a hetero ring structure known as a boroxazolidone. In this master’s thesis, the imine condensation between aldehydes and boroxazolidones, and the N-B bond were studied. -

1 Optimization of Salt Concentration and Explanation of Two Peak
Optimization of salt concentration and Explanation of Two Peak Percolation in Blend Solid Polymer Nanocomposite Films Anil Arya, A. L. Sharma* Centre for Physical Sciences, Central University of Punjab, Bathinda, Punjab-151001, INDIA E-mail: [email protected] Abstract The present paper report is focused toward the preparation of the flexible and freestanding blend solid polymer electrolyte films based on PEO-PVP complexed with NaPF6 by solution cast technique. The structural/morphological features of the synthesized polymer nanocomposite films have been investigated in detail using X-ray diffraction, Fourier transform infra-red spectroscopy, field emission scanning electron microscope, and atomic force microscopy + -6 -1 techniques. The film PEO-PVP+NaPF6 (Ö /Na = 8) exhibits highest ionic conductivity ~5.92×10 S cm at 40 °C and ~2.46×10-4 S cm-1 at 100 °C. The temperature dependent conductivity shows Arrhenius type behavior and activation energy decreases with the addition of salt. The high temperature (100 °C) conductivity monitoring is done + for the optimized PEO-PVP+NaPF6 (Ö /Na =8) highly conductive system and the conductivity is still maintained stable up to 160 h (approx. 7 days). The thermal transitions parameters were measured by the differential scanning calorimetry (DSC) measurements. The prepared polymer electrolyte film displays the smoother surface in addition of o salt and a thermal stability up to 300 C. The ion transference number (tion) for the highest conducting sample is found to be 0.997 and evidence that the present system is ion dominating with negligible electron contribution. Both linear sweep voltammetry and cyclic voltammetry supports the use of prepared polymer electrolyte with long-term cycle stability and thermal stability for the solid state sodium ion batteries. -

Chapter 7: Search for New Fire Suppressant Chemicals
Chapter 7: SEARCH FOR NEW FIRE J. Douglas Mather, Ph.D. SUPPRESSANT CHEMICALS Chemical Development Studies, Inc. Robert E. Tapscott, Ph.D. GlobeTech, Inc. TABLE OF CONTENTS 7.1 Fire Suppressant Replacement Knowledge Prior to the NGP .........................................612 7.1.1 Overview of Early Halon Replacement Efforts ....................................................612 7.1.2 Fire Suppressant Research – 1974 Through 1993.................................................613 7.1.3 DoD Technology Development Plan (1993 to 1997)............................................615 7.1.4 Advanced Agent Working Group (AAWG) .........................................................616 7.1.5 Summary: Alternative Agents and Selection Criteria Prior to the NGP ...............622 7.2 The NGP Approach to New Chemicals Screening..........................................................612 7.3 NGP Surveys of Inorganic Chemical Families................................................................612 7.3.1 Main Group Elements - Group I............................................................................626 7.3.2 Main Group Elements - Group II ..........................................................................627 7.3.3 Main Group Elements - Group III.........................................................................627 7.3.4 Main Group Elements - Group IV.........................................................................628 7.3.5 Main Group Elements - Group V..........................................................................634 -

Nuclear Magnetic Resonance Studies of Boron Trifluoride and Some of Its
NUCLEAR MAGNETIC R:~SONANCE S'I'UDH~S OF BORON TRIFLUORIDE AND SOME OF ITS COMPLEXES NUCLEAR MAGNETIC RESONANCE STUDIES OF BORON 'l1RIFLUORIDE AND SOME OF ITS COMPLEXES by JOI-frJ ST.2;PH_c;N HARTMAN, B.Sc. 1 M.Sc. A Thesis Submitted to the Faculty of Graduate Studies in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy McMaster University January, 1967 DOCTOR OF PHILOSOPHY (1967) McM.J'l.STER UNIVERSITY (Chemistry) Hamilton, Ontario. TITLE: Nuclear Magnetic Resonance Studies of Boron Trifluoride and Some of Its Complexes AUTHOR: John Stephen Hartman, B.Sc. (Queen's University), M.Sc. (University of Ottav,a) SUPVERVISOR: Professor R. J. Gillespie NUMB8R OF PJ\GES: xi, 231 SCOPE Al\JD com:ENTS: Nuclear magnetic resonance spectroscopy has been used to study donor'.'"acceptor complexes of boron trifluoride with ketones, water, and methanol. Various NMR techniques have been applied which, in certain favourable cases, hDve yielded information on the stoichiometry, struc ture, relative stabilities, and reactions of the complexes in soluUon. A new method is proposed for the detection of BF complexes having low 3 formation constants. The me.gni tude and sign of the B-F. coupling constant in the tetra fluoroborate anion have been found to be dependent on the solvent. This solvent dependence is interpreted in terms of salvation and association of the ion in solution. 19 10 F spectra of BF over a ranc;e of temperatures agree well 3 with line shapes calculated, using Pople's expressions, for coupling of fluorine-19 with boron-10 (1=3) which is undergoing partial quadru pole relaxation. -

Chapter 1: Introduction and Literature Review
Chapter 1: Introduction and Literature Review The International Energy Agency (IEA) reports that the world’s energy consumption has almost doubled between 1973 and 2013, from 5.42x107 kWh to 1.08x108 kWh [1], and as the demand for energy continues to increase it is estimated that by 2050 world energy supplies will have to double again to reach demand [2]. With climate change becoming an increasing issue, research continues to develop more efficient, safe and environmentally friendly methods to produce energy. It has been suggested by the IEA that nuclear and renewable energy are likely sources of clean energy in the future, and research is currently ongoing to allow this to happen [3]. The Generation IV International Forum (GIF), an international cooperative which aims to research and develop the next generation of nuclear reactors, have identified six different systems which they believe can achieve their four goal areas [4]: sustainability safety and reliability proliferation resistance and physical protection economic competitiveness The six reactor systems that were selected by the GIF in 2002 were: gas cooled fast reactor (GFR) lead cooled fast reactor (LFR) molten salt reactor (MSR) sodium cooled fast reactor (SFR) supercritical water-cooled reactor (SCWR) very high temperature reactor (VHTR) With continuing research, it is envisaged that each of these reactors will be able to produce energy while also attaining the original four goals [4]. The work in this thesis will focus on molten salt reactors, which produce energy in a unique way compared to the current operational fleet. One of the main research drivers is the corrosion resistance of the material of containment, as the fuel salt and coolant are highly corrosive in the presence of any impurities [5]. -

Selective Fluorination Strategies
Durham E-Theses Selective Fluorination Strategies BREEN, JESSICA,RUTH How to cite: BREEN, JESSICA,RUTH (2012) Selective Fluorination Strategies, Durham theses, Durham University. Available at Durham E-Theses Online: http://etheses.dur.ac.uk/3479/ Use policy The full-text may be used and/or reproduced, and given to third parties in any format or medium, without prior permission or charge, for personal research or study, educational, or not-for-prot purposes provided that: • a full bibliographic reference is made to the original source • a link is made to the metadata record in Durham E-Theses • the full-text is not changed in any way The full-text must not be sold in any format or medium without the formal permission of the copyright holders. Please consult the full Durham E-Theses policy for further details. Academic Support Oce, Durham University, University Oce, Old Elvet, Durham DH1 3HP e-mail: [email protected] Tel: +44 0191 334 6107 http://etheses.dur.ac.uk Abstract There is a great interest in the synthesis of fluorinated aromatic and heterocyclic compounds, which have a range of applications in the pharmaceutical industry. Many common routes to these compounds, however, are low yielding and or/expensive. This thesis is concerned with novel methods for the synthesis of fluoro-aromatics and fluoro- pyrazoles using conventional fluorinating agents, such as Selectfluor™, as well as using elemental fluorine and the flow reactor technology developed in Durham. Firstly, elemental fluorine was used to fluorinate a range of aromatics containing electron-donating substituents, using both batch and flow methods.