1056 T CELLS and AGING, JANUARY 2002 UPDATE Graham
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

I Harry Shapiro & Ann 3947 831 II II John R
#1 FROM BOOK GRAN'.fOR GRANTEE Book Page Jan 2 Sec, of H. & U.D. Mathias W. Guthrie & Nancy C. 3947 854 • II II II 3947 II II II 3947 II II ::: I Harry Shapiro & Ann 3947 831 II II John R. Siegel & Caroll A, 3947 965 II II Harry Shapiro & Ann 3947 909 I II II Anderson Williamson & Curlie Mae 3947 902 j II Schiedrich, Delores et al Noel E. Wynne, Jr., et al 3947 866 l ,! II Sentinel Realty Co., franklin D. JOnes & Betty 3947 858 ij II Schaefer, Portia F. Ben Schaefer Buildigg, Co., 3947 815 l II I I Straehley, Jr., Erwin (Dec'd) Cert of Tr Margaret C. Straeh l ey 3947 967 II Schnurr, Jr., Goerog L.et al William Arnold Breig & Janice C, 3947 953 I II Scott, Pete et al City of Cincinnati 3947 948. ~ II S to 11 , Lo i s 8 • Donald E. Julian & Mildred E. 3947 919 ~ II Stagge, Mary Agnes Bernard James Stagge 3947 I 899 ~ !1 II Schneider, Josef et a 1 Ferdinand A. Fo~ney, Trustee 3947 t 969 ril 3 Scott, Shelby (Dec'd) Cert of Tr. Edna Scott 3948 t • 74. JI II Sec, of H. & U.D. Ray Mjracle & Earleen M. 1• 3948 I 72 11 II 11 I 1 11 Ca r 1 J • Pa r ks 3948 l 211 Ji tl II 1 1 Mathias W. Guthrie & Nancy C. 157 1 ·~ 3948 1 !: II II II 3948 177. # ii II II I Carl J. Parks 3948 1 52 H ft II Scott, Robert 8, ii Betty Jo Scott 3948 92 j! II & Ji I Sec. -

Name Lebensdaten SS SM, Rosi
Name Lebensdaten Archivalien (Zeichen ≙ DSK-Sign. => MATERIALIEN) S S S. M., Rosi -MuM,D,Fi 19.. D 1.441,770 S., Anita -NaM 1895 D K1054 S., Ekatherina 19.. ART4.3-9 S., Johanna 19.. ART4.2 SA SA SA, Rosilda 19.. DS7 SA, Rosilda i335 SAAB, Jocelyne -Fi 1948 RL 1.411,779 SAAB, Jocelyne -Fi i3275 SAABYE, Mette -Ks 1969 DK K1430,1492 SAABYE, Susanne 1856-1939 DK Bu SAADE, Stephanie -I 1983 RL/F Be101,105,106 SAADEH, Raeda -P,F 1977 PS 6.144 SAADEH, Raeda -P,F Be39,41,42 SAADEH, Raeda -P,F i,i1289 SAADEH, Raeda -P,F K863,1062 SAADI, Hana al -P 19.. Q i6505 SAAD-SKOLNIK, Sabina -M 1950 I/IL 4.57.3 SAAGE, Carolin -F 1978 D i6636 SAAL, Linda Rosa -F 1985 D Be92 SAAL, Linda Rosa -F K1666 SAAL, Ulrike -Gl 1953 D K450 SAALFELD, Christine -G,NM,I 1968 D/NL Be59,61 SAALFELD, Katharina von -W 19.. F Be99 SAALFRANK, Gudrun 19.. K60 SAALMANN, Karin -B,M 1938-2005 D K2349 SAAM, Karin -G 1950 D K318,319 SAAMAN, Karen 19.. NL K1329.5 SAAR, Alison -O 1956 USA 1.253,423 SAAR, Alison -O Be110-113,115 SAAR, Betye -O 1926 USA 1.24,25,321,423,583 SAAR, Betye -O Be106,107,112-115 SAAR, Betye -O i (DK), SAAR, Betye -O P120 SAAR, Gabriele -M 1960 K1093,1094 SAAR, Katja -D 1984 D i3551,4761,4790 SAAR, Lezley -S 1953 USA 1.423 SAARBOURG, Irmtrud 1936 D KFBW SAARE, Mare -Kg 1965 EST Be71,82,86 SAARE, Mare -Kg i4795 SAARHELO, Sanna 19. -

(12) United States Patent (10) Patent No.: US 7,256.253 B2 Bridon Et Al
US00725.6253B2 (12) United States Patent (10) Patent No.: US 7,256.253 B2 Bridon et al. (45) Date of Patent: Aug. 14, 2007 (54) PROTECTION OF ENDOGENOUS 6,500,918 B2 12/2002 Ezrin et al. THERAPEUTIC PEPTIDES FROM 6,514,500 B1 2/2003 Bridon et al. PEPTIDASE ACTIVITY THROUGH 6,593,295 B2 7/2003 Bridon et al. CONUGATION TO BLOOD COMPONENTS 6,602,981 B2 8/2003 Ezrin et al. 6,610,825 B2 8, 2003 Ezrin et al. (75)75 Inventors: Dominique P. Bridon, San Francisco, 6,706,892 B1 3/2004 Ezrin et al. CA (US); Alan M. Ezrin, Moraga, CA (US); Peter G. Milner, Los Altos, CA 6,849,714 B1 2/2005 Bridon et al. (US); Darren L. Holmes, Anaheim, CA 2002fOO18751 A1 2/2002 Bridon et al. (US); Karen Thibaudeau, Rosemere 2003, OO73630 A1 4/2003 Bridon et al. (CA) 2003/O105867 A1 6/2003 Colrain et al. 2003. O108568 A1 6/2003 Bridon et al. (73) Assignee: Conjuchem Biotechnologies Inc., 2003/0170250 A1 9, 2003 EZrin et al. Montreal (CA) 2004/O127398 A1 7, 2004 Bridon et al. (*) Notice: Subject to any disclaimer, the term of this 2004/O138100 A1 7/2004 Bridon et al. patent is extended or adjusted under 35 2004/O156859 A1 8, 2004 Ezrin et al. U.S.C. 154(b) by 0 days. 2004/0248782 A1 12/2004 Bridon et al. 2004/0266673 Al 12/2004 Bakis et al. (21) Appl. No.: 11/066,697 2005, 0037974 A1 2/2005 Krantz et al. 2005, OO65075 A1 3, 2005 Erickson et al. -

Survey of Apple Clones in the United States
Historic, archived document Do not assume content reflects current scientific knowledge, policies, or practices. 5 ARS 34-37-1 May 1963 A Survey of Apple Clones in the United States u. S. DFPT. OF AGRffini r U>2 4 L964 Agricultural Research Service U.S. DEPARTMENT OF AGRICULTURE PREFACE This publication reports on surveys of the deciduous fruit and nut clones being maintained at the Federal and State experiment stations in the United States. It will b- published in three c parts: I. Apples, II. Stone Fruit. , UI, Pears, Nuts, and Other Fruits. This survey was conducted at the request of the National Coor- dinating Committee on New Crops. Its purpose is to obtain an indication of the volume of material that would be involved in establishing clonal germ plasm repositories for the use of fruit breeders throughout the country. ACKNOWLEDGMENT Gratitude is expressed for the assistance of H. F. Winters of the New Crops Research Branch, Crops Research Division, Agricultural Research Service, under whose direction the questionnaire was designed and initial distribution made. The author also acknowledges the work of D. D. Dolan, W. R. Langford, W. H. Skrdla, and L. A. Mullen, coordinators of the New Crops Regional Cooperative Program, through whom the data used in this survey were obtained from the State experiment stations. Finally, it is recognized that much extracurricular work was expended by the various experiment stations in completing the questionnaires. : CONTENTS Introduction 1 Germany 298 Key to reporting stations. „ . 4 Soviet Union . 302 Abbreviations used in descriptions .... 6 Sweden . 303 Sports United States selections 304 Baldwin. -

Monoclonal Antibody to Parathyroid Hormone / PTH (1-38) - Purified
OriGene Technologies, Inc. OriGene Technologies GmbH 9620 Medical Center Drive, Ste 200 Schillerstr. 5 Rockville, MD 20850 32052 Herford UNITED STATES GERMANY Phone: +1-888-267-4436 Phone: +49-5221-34606-0 Fax: +1-301-340-8606 Fax: +49-5221-34606-11 [email protected] [email protected] AM02147PU-N Monoclonal Antibody to Parathyroid hormone / PTH (1-38) - Purified Alternate names: Parathormone, Parathyrin Quantity: 0.1 mg Background: Parathyroid hormone (PTH), or Parathormone, is secreted by the parathyroid glands as a polypeptide containing 84 amino acids. It acts to increase the concentration of calcium in the blood, whereas calcitonin (a hormone produced by the parafollicular cells of the thyroid gland) acts to decrease calcium concentration. Uniprot ID: P01270 NCBI: NP_000306.1 GeneID: 5741 Host / Isotype: Mouse / IgG1 Recommended Isotype SM10P (for use in human samples), AM03095PU-N Controls: Clone: A1/70 Immunogen: Synthetic Human PTH (aa 1-38) poly-Lysine conjugated. AA Sequence: SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNFVALG Format: State: Lyophilized purified IgG fraction from Cell Culture Supernatant Purification: Protein G Chromatography Buffer System: PBS, pH 7.4 Reconstitution: Restore in aqua bidest to 1 mg/ml Applications: RIA: 20 ng/ml. ELISA: 1 µg/ml (Ref.1). ILMA: 20 µg/ml (Ref.3). Immunohistochemistry on Cryosections and Paraffin Sections: 2 µg/ml. Other applications not tested. Optimal dilutions are dependent on conditions and should be determined by the user. Specificity: This antibody detects PTH peptide (aa 15-25; 1-34; 1-38; 1-84; 7-84). There were no cross reactivities obtained with synthetic Human PTH (aa 1-3; 1-10; 4-16; 28-48; 39-84; 44-68; 53-84) nor with PTHrP (aa 1-86), Calcitonin, Gastrin, Beta-2 Microglobulin, Thymulin, Thyroglobulin, Streptavidin, or Glutathione S-transferase. -

Activity of the Pineal Gland, Thymus and Hypophysial- Adrenal System in Oncological Patients I.F
138 Experimental Oncology 25, 138-142, 2003 (June) ACTIVITY OF THE PINEAL GLAND, THYMUS AND HYPOPHYSIAL- ADRENAL SYSTEM IN ONCOLOGICAL PATIENTS I.F. Labunets*, Yu.A. Grinevich Institute of Oncology, Academy of Medical Sciences of Ukraine, Kyiv 03022, Ukraine ÀÊÒÈÂÍÎÑÒÜ ÝÏÈÔÈÇÀ, ÒÈÌÓÑÀ È ÃÈÏÎÔÈÇÀÐÍÎ- ÍÀÄÏÎ×Å×ÍÈÊÎÂÎÉ ÑÈÑÒÅÌÛ Ó ÁÎËÜÍÛÕ ÎÍÊÎËÎÃÈ×ÅÑÊÎÃÎ ÏÐÎÔÈËß È.Ô. Ëàáóíåö*, Þ.À. Ãðèíåâè÷ Èíñòèòóò îíêîëîãèè ÀÌÍ Óêðàèíû, Êèåâ, Óêðàèíà Melatonin, thymic serum factor (FTS), alpha-melanocytestimulating hormone (alpha-MSH) and cortisol levels in blood serum and urine of healthy subjects and patients with skin melanoma and malignant thymoma of different age groups have been studied. It has been found that in healthy 20–29 year old men the highest melatonin level was observed in winter, those of FTS, cortisol and alpha-MSH — in summer-autumn, autumn-winter and summer, respectively. In male induviduals over 30 years, the increase of melatonin level in winter was not registered, and in those over 40, the stable secretion of FTS and cortisol and decrease of alpha-MSH acrophase at spring time were observed. In healthy women under 40, melatonin level was heightened in follicular and luteal phase of cycle and that of FTS — in luteal phase. Stability of melatonin secretion and reduction of FTS content in luteal phase of cycle were typical for women over 40. The age-related disorders of indices were more pronounced upon tumor development. In men under 40 years suffering from melanoma and thymoma, circannual changes of pineal gland, thymus and hypophysial-adrenal system function were typical for healthy subjects after 40 years. In women with melanoma and thymoma under 40 years melatonin and FTS level during menstrual cycle were similar with those in healthy women over 40. -

Sackler Faculty of Medicine Clinical
Sackler Faculty of Medicine Clinical Research 2017 בעקבות הלא נודע בעקבות הלא נודע Sections Cancer 6 Cardiovascular System 45 Digestive System 72 Endocrine Disease 88 Genetic Diseases & Genomics 107 Immunology & Hematology 127 Infectious Diseases 133 Neurological & Psychiatric Diseases 144 Ophthalmology 181 Public Health 187 Reproduction 198 Stem Cells & Regenerative Medicine 205 Cover images (from bottom left, clockwise): Image 1: Staining of a novel anti-frizzled7 monoclonal antibody directed at tumor stem Cells. Credit: Benjamin Dekel lab. Image 2: Growing adult kidney spheroids and organoids for cell therapy. Credit: Benjamin Dekel lab. Image 3 & 4: Vibrio proteolyticus bacteria infecting macrophages. Credit: Dor Salomon. Image 5: K562 leukemia cells responding to complement attack (red-complement C9, green-mitochondrial stress protein mortalin) Credit: Niv Mazkereth, Zvi Fishelson. Image 6: Cardiomyocyte proliferation in newborn mouse heart by phosphohistone 3 staining (purple). Credit: Jonathan Leor. © All rights reserved Editor: Prof. Karen Avraham Graphic design: Michal Semo Kovetz, TAU Graphic Design Studio June 2017 Sackler Faculty of Medicine Research 2017 2 בעקבות הלא נודע The Sackler Faculty of Medicine The Sackler Faculty of Medicine is Israel’s largest diabetes, neurodegenerative diseases, infectious medical research and training complex. The Sackler diseases and genetic diseases, including but not Faculty of Medicine of Tel Aviv University (TAU) was imited to Alzheimer’s disease, Parkinson’s disease founded in 1964 following the generous contributions and HIV/AIDS. Physicians in 181 Sacker affiliated of renowned U.S. doctors and philanthropists departments and institutes in 17 hospitals hold Raymond, and the late Mortimer and Arthur Sackler. academic appointments at TAU. The Gitter-Smolarz Research at the Sackler Faculty of Medicine is Life Sciences and Medicine Library serves students multidisciplinary, as scientists and clinicians combine and staff and is the center of a consortium of 15 efforts in basic and translational research. -

Studies on Some Apple Virus Diseases in New Hampshire Joseph G
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Spring 1958 STUDIES ON SOME APPLE VIRUS DISEASES IN NEW HAMPSHIRE JOSEPH G. BARRAT Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation BARRAT, JOSEPH G., "STUDIES ON SOME APPLE VIRUS DISEASES IN NEW HAMPSHIRE" (1958). Doctoral Dissertations. 752. https://scholars.unh.edu/dissertation/752 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. Dapple apple symptoms on the fruits of the variety Starking. STUDIES ON SOME APPLE VIRUS DISEASES IN NEW HAMPSHIRE By Joseph G. Barrat B. S., Rhode Island State College, 19*+8 M. S., University of Rhode Island, 1951 A DISSERTATION Submitted to the University of New Hampshire In Partial Fulfillment of The Requirements for the Degree of Doctor of Philosophy Graduate School Department of Botany May, 1958 This dissertation has been examined and approved. ■?. / T Date ACKNOWLEDGMENTS The writer wishes to express his deep appreciation to Dr. Avery E. Rich for his assistance and permission to develop the study along those lines which seemed most opportune. The writer is indebted to Dr. Albion R. Hodgdon for his taxonomic assistance, Dr. Stuart Dunn for permis sion to use the available space in the light room, Dr, R. A. Kilpatrick for help with the photographs and Dr. W. -

Äppelklonarkivet I Bergianska Trädgården
Äppelklonarkivet i Bergianska trädgården P. J. Bergius Äppelklonarkivet Bergianska trädgårdens klonarkiv startades 1981 av dåvarande Professor Bergianus Måns Ryberg som var engagerad i Nordiska Genbanken för frukt och bär. Ett 30-tal sorter planterades från början och har genom åren utökats. Idag ingår klonarkiven i verksamheten vid Programmet för Odlad Mångfald (POM). Anledningen till att upprätta denna typ av klonarkiv är att bevara en mångfald av gamla kulturväxter. Modern växtförädling kan innebära framställning av några få men högproducerande sorter. Dessa tenderar att ersätta mångfalden av äldre lokala sorter, som tillsammans utgjort en stor genetisk variation för olika egenskaper. Om man har ett fåtal sorter blir dessa lätt mottagliga för växtsjukdomar. Därför är det viktigt att bevara ett större urval av gammalt sortmaterial som kan behövas för framtida växtförädling. När det gäller de odlade växterna finns dessutom ett stort kulturhisto- riskt värde i att bevara gamla lokala sorter. Dessa kan ha haft stor bety- delse för människors försörjning och spelat en betydande roll i en bygds utveckling. Sorter av äpplen och päron hålls vid liv genom så kallad vegetativ förök- ning. Ursprungligen har varje sort uppstått ur en kärna. Men vill man bevara en speciell sort med dess speciella egenskaper så måste detta ske genom exempelvis ympning. Vid kärnsådd uppstår alltid nya typer. Vid växtförädling av äpplen görs korsningar där båda föräldrarna är kända. Sedan sås en stor mängd kärnor av denna korsning och man får då förhoppningsvis några plantor som ger bra äpplen med nya egenskaper värda att bevara som en ny sort. Det tar 20–30 år att få fram en ny äp- pelsort från korsning till att den finns tillgänglig i handeln. -
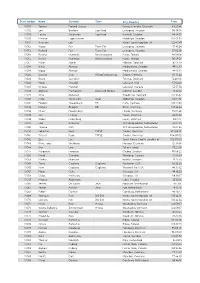
Start Number Name Surname Team City, Country Time 11001 Tommy
Start number Name Surname Team City, Country Time 11001 Tommy Frølund Jensen Kongens lyngby, Denmark 89:20:46 11002 Emil Boström Lag Nord Linköping, Sweden 98:19:04 11003 Lovisa Johansson Lag Nord Burträsk, Sweden 98:19:02 11005 Maurice Lagershausen Huddinge, Sweden 102:01:54 11011 Hyun jun Lee Korea, (south) republic of 122:40:49 11012 Viggo Fält Team Fält Linköping, Sweden 57:45:24 11013 Richard Fält Team Fält Linköping, Sweden 57:45:28 11014 Rasmus Nummela Rackmannarna Pjelax, Finland 98:59:06 11015 Rafael Nummela Rackmannarna Pjelax, Finland 98:59:06 11017 Marie Zølner Hillerød, Denmark 82:11:49 11018 Niklas Åkesson Mellbystrand, Sweden 99:45:22 11019 Viggo Åkesson Mellbystrand, Sweden 99:45:22 11020 Damian Sulik #StopComplaining Siegen, Germany 101:13:24 11023 Bruno Svendsen Tylstrup, Denmark 74:48:08 11024 Philip Oswald Lakeland, USA 55:52:02 11025 Virginia Marshall Lakefield, Canada 55:57:54 11026 Andreas Mathiasson Backyard Heroes Ödsmål, Sweden 75:12:02 11028 Stine Andersen Brædstrup, Denmark 104:35:37 11030 Patte Johansson Tiger Södertälje, Sweden 80:24:51 11031 Frederic Wiesenbach BB Dahn, Germany 101:21:50 11032 Romea Brugger BB Berlin, Germany 101:22:44 11034 Oliver Freudenberg Heyda, Germany 75:07:48 11035 Jan Hennig Farum, Denmark 82:11:50 11036 Robert Falkenberg Farum, Denmark 82:11:51 11037 Edo Boorsma Sint annaparochie, Netherlands 123:17:34 11038 Trijneke Stuit Sint annaparochie, Netherlands 123:17:35 11040 Sebastian Keck TATSE Gieäen, Germany 104:08:01 11041 Tatjana Kage TATSE Gieäen, Germany 104:08:02 11042 Eun Lee -

MINIREVIEWS Central Nervous System-Immune System Interactions: Psychoneuroendocrinology of Stress and Its Immune Consequences PAUL H
ANTIMICROBIAL AGENTS AND CHEMOTHERAPY, Jan. 1994, p. 1-6 Vol. 38, No. 1 0066-4804/94/$04.OO+O Copyright X 1994, American Society for Microbiology MINIREVIEWS Central Nervous System-Immune System Interactions: Psychoneuroendocrinology of Stress and Its Immune Consequences PAUL H. BLACK* Department ofMicrobiology, Boston University School ofMedicine, Boston, Massachusetts 02118 The past 20 years has witnessed the emergence of the field receiving information from the periphery, integrating it with of psychoneuroimmunology (48). This field deals with the the internal environment, and adjusting certain functions influence of the central nervous system (CNS) on the im- such as sympathetic nervous system function and endocrine mune system, or more specifically, whether and how secretion (28). The hypothalamus influences the pituitary thoughts and emotions affect immune function. Studies have gland through a variety of polypeptide "releasing factors," concentrated, for the most part, on the effects of stress on for example, corticotropin-releasing factor (CRF), which the immune system. Stress is defined as a state of dishar- controls the release of corticotropin (ACTH) from the ante- mony or threatened homeostasis provoked by a psycholog- rior pituitary gland. Other hypothalamic releasing hormones ical, environmental, or physiologic stressor (12, 40). It has (RHs) include thyrotropin RH, growth hormone RH, and also become apparent from these studies that the immune luteinizing hormone RH; these control the release of thyro- system can influence the CNS, and thus, a circuit exists tropin, growth hormone, gonadotropin, and luteinizing hor- between these two systems. Regulatory molecules or cyto- mone from the anterior pituitary gland. In addition, hypo- kines elaborated from activated immune cells evoke a thalamic somatostatin and dopamine inhibit the release of CNS response which, in turn, affects the immune system growth hormone and prolactin, respectively, from the ante- (26). -

11695003.Pdf
CORE Metadata, citation and similar papers at core.ac.uk Provided by Epsilon Open Archive ACTA UNIVERSITATIS AGRICULTURAE SUECIAE Control of Pre- and Postharvest Factors to Improve Apple Quality and Storability Ibrahim Tahir Faculty of Landscape Planning, Horticulture and Agricultural Science Department of Crop Science- Alnarp Doctoral thesis 2006:35 Swedish University of Agricultural Sciences Control of Pre- and Postharvest Factors to Improve Apple Quality and Storability Ibrahim Tahir Faculty of Landscape Planning, Horticulture and Agricultural Science Department of Crop Sciences- Alnarp Doctoral thesis Swedish University of Agricultural Sciences Alnarp 2006 Acta Universitatis Agriculturae Sueciae Year: 2006:35 ISSN 1652-6880 ISBN 91-576-7084-6 © 2006 Ibrahim Tahir Tryck: SLU Service/Repro, Alnarp 2006 Abstract Tahir, I. I. 2006. Control of Pre- and Postharvest Factors to Improve Fruit Quality and Storability. Doctoral dissertation. ISSN 1652-6880, ISBN 91-576-7084-6 To increase apple production in Sweden and to improve apple quality and competitive edge on the market, solutions to negative problems such as bruising susceptibility, decay and weak storage potential have to be discovered. This thesis investigated problems relating to apple quality and storability, based on the results of field and laboratory experiments conducted in Kivik, Sweden, during a period of 12 years. Four cultivars (Aroma, Ingrid Marie, Cox’s Orange Pippin, and Katja) were predominantly used for the investigations. The cultivar Aroma showed the highest internal phenol content and was the most sensitive to bruising, followed by cv. Ingrid Marie. Enhancement of water loss and melting of skin wax by air pre-cooling or post-harvest heating decreased the incidence of bruising.