Cvscaremark ® Maintenance Drug List
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Prospective Study on Gynaecological Effects of Two Antioestrogens Tamoxifen and Toremifene in Postmenopausal Women
British Journal of Cancer (2001) 84(7), 897–902 © 2001 Cancer Research Campaign doi: 10.1054/ bjoc.2001.1703, available online at http://www.idealibrary.com on http://www.bjcancer.com Prospective study on gynaecological effects of two antioestrogens tamoxifen and toremifene in postmenopausal women MB Marttunen1, B Cacciatore1, P Hietanen2, S Pyrhönen2, A Tiitinen1, T Wahlström3 and O Ylikorkala1 1Departments of Obstetrics and Gynecology, Helsinki University Central Hospital, P.O. Box 140, FIN-00029 HYKS, Finland; 2Department of Oncology, Helsinki University Central Hospital, P.O. Box 180, FIN-00029 HYKS, Finland; 3Department of Pathology, Helsinki University Central Hospital, P.O. Box 140, FIN-00029 HYKS, Finland Summary To assess and compare the gynaecological consequences of the use of 2 antioestrogens we examined 167 postmenopausal breast cancer patients before and during the use of either tamoxifen (20 mg/day, n = 84) or toremifene (40 mg/day, n = 83) as an adjuvant treatment of stage II–III breast cancer. Detailed interview concerning menopausal symptoms, pelvic examination including transvaginal sonography (TVS) and collection of endometrial sample were performed at baseline and at 6, 12, 24 and 36 months of treatment. In a subgroup of 30 women (15 using tamoxifen and 15 toremifene) pulsatility index (PI) in an uterine artery was measured before and at 6 and 12 months of treatment. The mean (±SD) follow-up time was 2.3 ± 0.8 years. 35% of the patients complained of vasomotor symptoms before the start of the trial. This rate increased to 60.0% during the first year of the trial, being similar among patients using tamoxifen (57.1%) and toremifene (62.7%). -

Contraception and Misconceptions
CONTRACEPTION AND MISCONCEPTIONS CONTRACEPTION IN WOMEN WITH MENTAL ILLNESS OVERVIEW Hormones and mood How mental illness impacts on contraceptive choice Ideal contraception Pro and cons of contraceptive methods in women with mental illness Hormonal contraception and mood ESTROGENS anti-inflammatory neuroprotective effects of estradiol modulation of the limbic processing memory of emotionally-relevant information. estradiol “beneficially” modulates pathways implicated in the pathophysiology of depression, including serotonin and norepinephrine pathways PROGESTROGENS Previously thought to be anxiogenic, depressogenic Breakdown products Depression – alpha hydroxy progestrogen breakdown products pro – inflammatory, anxiogenic HORMONES AND MOODS – COMPLEX INTERPLAY MENTAL ILLNESS AND CONTRACEPTIVE CHOICE Effect of mental illness on contraceptive choice Effect of contraceptive choice on mental illness Drug interactions EFFECT MENTAL ILLNESS ON CONTRACEPTIVE CHOICE Impulsive Poor planning Cognitive and problem judgement Poor adherence IMPULSIVITY COGNITION POOR JUDGEMENT LETS NOT FORGET SUBSTANCES…… TYPES OF CONTRACEPTION No method Non hormonal Condom/Femidom Diaphragm Non hormone containing IUCD (Copper T) Hormonal COC POP Mirena Evra Patch Nuva Ring Implant ORAL CONTRACEPTIVES POP – avoid Same time every day COC Pros Effective Reduction PMS – monophasic estrogen dominant pill eg Femodene, Yaz, Nordette Cons Drug interactions Pill burden Daily dose EVRA PATCH Weekly patch Transdermal system: 150 mcg/day -

209627Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 209627Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review Reviewers of Multi-Disciplinary Review and Evaluation SECTIONS OFFICE/ AUTHORED/ ACKNOWLEDGED/ DISCIPLINE REVIEWER DIVISION APPROVED Mark Seggel, Ph.D. OPQ/ONDP/DNDP2 Authored: Section 4.2 Digitally signed by Mark R. Seggel -S CMC Lead DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, cn=Mark R. Signature: Mark R. Seggel -S Seggel -S, 0.9.2342.19200300.100.1.1=1300071539 Date: 2018.08.08 16:29:15 -04'00' Frederic Moulin, DVM, PhD OND/ODE3/DBRUP Authored: Section 5 Pharmacology/ Digitally signed by Frederic Moulin -S Toxicology DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Reviewer Signature: Frederic Moulin -S 0.9.2342.19200300.100.1.1=2001708658, cn=Frederic Moulin -S Date: 2018.08.08 15:26:57 -04'00' Kimberly Hatfield, PhD OND/ODE3/DBRUP Approved: Section 5 Pharmacology/ Toxicology Digitally signed by Kimberly P. Hatfield -S DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Team Leader Signature: Kimberly P. Hatfield -S 0.9.2342.19200300.100.1.1=1300387215, cn=Kimberly P. Hatfield -S Date: 2018.08.08 14:56:10 -04'00' Li Li, Ph.D. OCP/DCP3 Authored: Sections 6 and 17.3 Clinical Pharmacology Dig ta ly signed by Li Li S DN c=US o=U S Government ou=HHS ou=FDA ou=People Reviewer cn=Li Li S Signature: Li Li -S 0 9 2342 19200300 100 1 1=20005 08577 Date 2018 08 08 15 39 23 04'00' Doanh Tran, Ph.D. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -

U.S. Medical Eligibility Criteria for Contraceptive Use, 2016
Morbidity and Mortality Weekly Report Recommendations and Reports / Vol. 65 / No. 3 July 29, 2016 U.S. Medical Eligibility Criteria for Contraceptive Use, 2016 U.S. Department of Health and Human Services Centers for Disease Control and Prevention Recommendations and Reports CONTENTS Introduction ............................................................................................................1 Methods ....................................................................................................................2 How to Use This Document ...............................................................................3 Keeping Guidance Up to Date ..........................................................................5 References ................................................................................................................8 Abbreviations and Acronyms ............................................................................9 Appendix A: Summary of Changes from U.S. Medical Eligibility Criteria for Contraceptive Use, 2010 ...........................................................................10 Appendix B: Classifications for Intrauterine Devices ............................. 18 Appendix C: Classifications for Progestin-Only Contraceptives ........ 35 Appendix D: Classifications for Combined Hormonal Contraceptives .... 55 Appendix E: Classifications for Barrier Methods ..................................... 81 Appendix F: Classifications for Fertility Awareness–Based Methods ..... 88 Appendix G: Lactational -

Recommendations for Contraceptive Use, 2013 Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2Nd Edition
Morbidity and Mortality Weekly Report Early Release / Vol. 62 June 14, 2013 U.S. Selected Practice Recommendations for Contraceptive Use, 2013 Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2nd Edition Continuing Education Examination available at http://www.cdc.gov/mmwr/cme/conted.html. U.S. Department of Health and Human Services Centers for Disease Control and Prevention Early Release CONTENTS CONTENTS (Continued) Introduction ............................................................................................................1 Appendix A: Summary Chart of U.S. Medical Eligibility Criteria for Methods ....................................................................................................................2 Contraceptive Use, 2010 .................................................................................. 47 How To Use This Document ...............................................................................3 Appendix B: When To Start Using Specific Contraceptive Summary of Changes from WHO SPR ............................................................4 Methods .............................................................................................................. 55 Contraceptive Method Choice .........................................................................4 Appendix C: Examinations and Tests Needed Before Initiation of Maintaining Updated Guidance ......................................................................4 Contraceptive Methods -
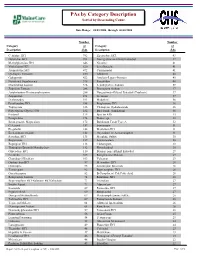
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Printed Formulary Catalog Basic
Scripps Health Formulary July 2016 Foreword Pharmacy and Therapeutics Committee. MedImpact approves such multi- This document represents the efforts of the MedImpact Healthcare Systems source drugs for addition to the MAC list based on the following criteria: Pharmacy and Therapeutics (P & T) and Formulary Committees to provide physicians A multi-source drug product manufactured by at least one (1) nationally and pharmacists with a method to evaluate the safety, efficacy and cost-effectiveness marketed company. of commercially available drug products. A structured approach to the drug selection At least one (1) of the generic manufacturer’s products must have an “A” process is essential in ensuring continuing patient access to rational drug therapies. rating or the generic product has been determined to be unassociated with The ultimate goal of the Portfolio Formulary is to provide a process and framework to efficacy, safety or bioequivalency concerns by the MedImpact P & T support the dynamic evolution of this document to guide prescribing decisions that Committee. reflect the most current clinical consensus associated with drug therapy decisions. Drug product will be approved for generic substitution by the MedImpact P & T Committee. This is accomplished through the auspices of the MedImpact P & T and Formulary Committees. These committees meet quarterly and more often as warranted to ensure This list is reviewed and updated periodically based on the clinical literature and clinical relevancy of the Formulary. To accommodate changes to this document, pharmacokinetic characteristics of currently available versions of these drug updates are made accessible as necessary. products. As you use this Formulary, you are encouraged to review the information and provide If a member or physician requests a brand name product in lieu of an approved your input and comments to the MedImpact P & T and Formulary Committees. -

Ribociclib (LEE011)
Clinical Development Ribociclib (LEE011) Oncology Clinical Protocol CLEE011G2301 (EarLEE-1) / NCT03078751 An open label, multi-center protocol for U.S. patients enrolled in a study of ribociclib with endocrine therapy as an adjuvant treatment in patients with hormone receptor-positive, HER2- negative, high risk early breast cancer Authors Document type Amended Protocol Version EUDRACT number 2014-001795-53 Version number 02 (Clean) Development phase II Document status Final Release date 17-Apr-2018 Property of Novartis Confidential May not be used, divulged, published or otherwise disclosed without the consent of Novartis Template version 22-Jul-2016 Novartis Confidential Page 2 Amended Protocol Version 02 (Clean) Protocol No. CLEE011G2301 Table of contents Table of contents ................................................................................................................. 2 List of tables ........................................................................................................................ 5 List of abbreviations ............................................................................................................ 6 Glossary of terms ................................................................................................................. 9 Protocol summary .............................................................................................................. 10 Amendment 2 (17-Apr-2018) ............................................................................................ 14 -

USP Reference Standards Catalog
Last Updated On: January 6, 2016 USP Reference Standards Catalog Catalog # Description Current Lot Previous Lot CAS # NDC # Unit Price Special Restriction 1000408 Abacavir Sulfate R028L0 F1L487 (12/16) 188062-50-2 $222.00 (200 mg) 1000419 Abacavir Sulfate F0G248 188062-50-2 $692.00 Racemic (20 mg) (4-[2-amino-6-(cyclo propylamino)-9H-pur in-9yl]-2-cyclopenten e-1-methanol sulfate (2:1)) 1000420 Abacavir Related F1L311 F0H284 (10/13) 124752-25-6 $692.00 Compound A (20 mg) ([4-(2,6-diamino-9H- purin-9-yl)cyclopent- 2-enyl]methanol) 1000437 Abacavir Related F0M143 N/A $692.00 Compound D (20 mg) (N6-Cyclopropyl-9-{( 1R,4S)-4-[(2,5-diami no-6-chlorpyrimidin- 4-yloxy)methyl] cyclopent-2-enyl}-9H -purine-2,6-diamine) 1000441 Abacavir Related F1L318 F0H283 (10/13) N/A $692.00 Compound B (20 mg) ([4-(2,5-diamino-6-c Page 1 Last Updated On: January 6, 2016 USP Reference Standards Catalog Catalog # Description Current Lot Previous Lot CAS # NDC # Unit Price Special Restriction hloropyrimidin-4-yla mino)cyclopent-2-en yl]methanol) 1000452 Abacavir Related F1L322 F0H285 (09/13) 172015-79-1 $692.00 Compound C (20 mg) ([(1S,4R)-4-(2-amino -6-chloro-9H-purin-9 -yl)cyclopent-2-enyl] methanol hydrochloride) 1000485 Abacavir Related R039P0 F0J094 (11/16) N/A $692.00 Compounds Mixture (15 mg) 1000496 Abacavir F0J102 N/A $692.00 Stereoisomers Mixture (15 mg) 1000500 Abacavir System F0J097 N/A $692.00 Suitability Mixture (15 mg) 1000521 Acarbose (200 mg) F0M160 56180-94-0 $222.00 (COLD SHIPMENT REQUIRED) 1000532 Acarbose System F0L204 N/A $692.00 Suitability -

Toremifene (Fareston)
Clinical Policy: Toremifene (Fareston) Reference Number: PA.CP.PMN.126 Effective Date: 10.17.18 Last Review Date: 04.17.19 Revision Log Description Toremifene (Fareston®) is an estrogen agonist/antagonist. FDA Approved Indication(s) Fareston is indicated for the treatment of metastatic breast cancer in postmenopausal women with estrogen-receptor positive or unknown tumors. Policy/Criteria Provider must submit documentation (such as office chart notes, lab results or other clinical information) supporting that member has met all approval criteria. It is the policy of health plans affiliated with PA Health & Wellness® that Fareston is medically necessary when the following criteria are met: I. Initial Approval Criteria A. Breast Cancer (must meet all): 1. Diagnosis of recurrent or metastatic breast cancer; 2. Prescribed by or in consultation with an oncologist; 3. Failure of a 1-month trial of tamoxifen at up to maximally indicated doses, unless contraindicated or clinically significant adverse effects are experienced; 4. Failure of a 1-month trial of an aromatase inhibitor (e.g., anastrozole, exemestane, letrozole) at up to maximally indicated doses, unless contraindicated or clinically significant adverse effects are experienced (see Appendix B); 5. Request meets one of the following (a or b): a. Dose does not exceed 60 mg per day (1 tablet/day); b. Dose is supported by practice guidelines or peer-reviewed literature for the relevant off-label use (prescriber must submit supporting evidence). Approval duration: 12 months B. Soft Tissue Sarcoma - Desmoid Tumors (off-label) (must meet all): 1. Diagnosis of desmoid tumor or aggressive fibromatosis; 2. Prescribed by or in consultation with an oncologist; 3. -

Effects of Toremifene, a Selective Estrogen Receptor Modulator, On
ISSN 2472-1972 Effects of Toremifene, a Selective Estrogen Receptor Modulator, on Spontaneous and Stimulated GH Secretion, IGF-I, and IGF-Binding Proteins in Healthy Elderly Subjects Ferdinand Roelfsema,1 Rebecca J. Yang,2 Paul Y. Takahashi,3 Dana Erickson,4 Cyril Y. Bowers,5 and Johannes D. Veldhuis2 1Department of Internal Medicine, Section of Endocrinology and Metabolism, Leiden University Medical Center, 2333 ZA Leiden, Netherlands; 2Endocrine Research Unit, Mayo School of Graduate Medical Education, Center for Translational Science Activities, Mayo Clinic, Rochester, Minnesota 55905; 3Department of Primary Care Internal Medicine, Mayo Clinic, Rochester, Minnesota 55905; 4Department of Endocrinology, Mayo Clinic, Rochester, Minnesota 55905; and 5Department of Internal Medicine, Tulane University Health Sciences Center, New Orleans, Louisiana 70112 Context: Estrogens amplify spontaneous and stimulated growth hormone (GH) secretion, whereas they diminish GH-dependent insulin-like growth factor (IGF)-I in a dose-dependent manner. Selective es- trogen receptor modulators (SERMs), including tamoxifen and toremifene, are widely adjunctively used in breast and prostate cancer. Although some endocrine effects of tamoxifen are known, few data are available for toremifene. Objective: To explore sex-dependent effects of toremifene on spontaneous 10-hour overnight GH se- cretion, followed by GH-releasing hormone–ghrelin stimulation. Additionally, effects on IGF-I, its binding proteins, and sex hormone–binding globulin (SHBG) were quantified. Participants and Design: Twenty men and 20 women, within an allowableagerangeof50to80years, volunteered for this double-blind, placebo-controlled prospective crossover study. Ten-minute blood sampling was done for 10 hours overnight and then for 2 hours after combined GH-releasing hormone–ghrelin injection. Main Outcome Measures: Pulsatile GH and stimulated GH secretion, and fasting levels of IGF-I, IGF- binding protein (IGFBP)1, IGFBP3, and SHBG.