Local Health Department Formulary for Nurse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Contraception and Misconceptions
CONTRACEPTION AND MISCONCEPTIONS CONTRACEPTION IN WOMEN WITH MENTAL ILLNESS OVERVIEW Hormones and mood How mental illness impacts on contraceptive choice Ideal contraception Pro and cons of contraceptive methods in women with mental illness Hormonal contraception and mood ESTROGENS anti-inflammatory neuroprotective effects of estradiol modulation of the limbic processing memory of emotionally-relevant information. estradiol “beneficially” modulates pathways implicated in the pathophysiology of depression, including serotonin and norepinephrine pathways PROGESTROGENS Previously thought to be anxiogenic, depressogenic Breakdown products Depression – alpha hydroxy progestrogen breakdown products pro – inflammatory, anxiogenic HORMONES AND MOODS – COMPLEX INTERPLAY MENTAL ILLNESS AND CONTRACEPTIVE CHOICE Effect of mental illness on contraceptive choice Effect of contraceptive choice on mental illness Drug interactions EFFECT MENTAL ILLNESS ON CONTRACEPTIVE CHOICE Impulsive Poor planning Cognitive and problem judgement Poor adherence IMPULSIVITY COGNITION POOR JUDGEMENT LETS NOT FORGET SUBSTANCES…… TYPES OF CONTRACEPTION No method Non hormonal Condom/Femidom Diaphragm Non hormone containing IUCD (Copper T) Hormonal COC POP Mirena Evra Patch Nuva Ring Implant ORAL CONTRACEPTIVES POP – avoid Same time every day COC Pros Effective Reduction PMS – monophasic estrogen dominant pill eg Femodene, Yaz, Nordette Cons Drug interactions Pill burden Daily dose EVRA PATCH Weekly patch Transdermal system: 150 mcg/day -

209627Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 209627Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review Reviewers of Multi-Disciplinary Review and Evaluation SECTIONS OFFICE/ AUTHORED/ ACKNOWLEDGED/ DISCIPLINE REVIEWER DIVISION APPROVED Mark Seggel, Ph.D. OPQ/ONDP/DNDP2 Authored: Section 4.2 Digitally signed by Mark R. Seggel -S CMC Lead DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, cn=Mark R. Signature: Mark R. Seggel -S Seggel -S, 0.9.2342.19200300.100.1.1=1300071539 Date: 2018.08.08 16:29:15 -04'00' Frederic Moulin, DVM, PhD OND/ODE3/DBRUP Authored: Section 5 Pharmacology/ Digitally signed by Frederic Moulin -S Toxicology DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Reviewer Signature: Frederic Moulin -S 0.9.2342.19200300.100.1.1=2001708658, cn=Frederic Moulin -S Date: 2018.08.08 15:26:57 -04'00' Kimberly Hatfield, PhD OND/ODE3/DBRUP Approved: Section 5 Pharmacology/ Toxicology Digitally signed by Kimberly P. Hatfield -S DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Team Leader Signature: Kimberly P. Hatfield -S 0.9.2342.19200300.100.1.1=1300387215, cn=Kimberly P. Hatfield -S Date: 2018.08.08 14:56:10 -04'00' Li Li, Ph.D. OCP/DCP3 Authored: Sections 6 and 17.3 Clinical Pharmacology Dig ta ly signed by Li Li S DN c=US o=U S Government ou=HHS ou=FDA ou=People Reviewer cn=Li Li S Signature: Li Li -S 0 9 2342 19200300 100 1 1=20005 08577 Date 2018 08 08 15 39 23 04'00' Doanh Tran, Ph.D. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -

U.S. Medical Eligibility Criteria for Contraceptive Use, 2016
Morbidity and Mortality Weekly Report Recommendations and Reports / Vol. 65 / No. 3 July 29, 2016 U.S. Medical Eligibility Criteria for Contraceptive Use, 2016 U.S. Department of Health and Human Services Centers for Disease Control and Prevention Recommendations and Reports CONTENTS Introduction ............................................................................................................1 Methods ....................................................................................................................2 How to Use This Document ...............................................................................3 Keeping Guidance Up to Date ..........................................................................5 References ................................................................................................................8 Abbreviations and Acronyms ............................................................................9 Appendix A: Summary of Changes from U.S. Medical Eligibility Criteria for Contraceptive Use, 2010 ...........................................................................10 Appendix B: Classifications for Intrauterine Devices ............................. 18 Appendix C: Classifications for Progestin-Only Contraceptives ........ 35 Appendix D: Classifications for Combined Hormonal Contraceptives .... 55 Appendix E: Classifications for Barrier Methods ..................................... 81 Appendix F: Classifications for Fertility Awareness–Based Methods ..... 88 Appendix G: Lactational -

Recommendations for Contraceptive Use, 2013 Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2Nd Edition
Morbidity and Mortality Weekly Report Early Release / Vol. 62 June 14, 2013 U.S. Selected Practice Recommendations for Contraceptive Use, 2013 Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2nd Edition Continuing Education Examination available at http://www.cdc.gov/mmwr/cme/conted.html. U.S. Department of Health and Human Services Centers for Disease Control and Prevention Early Release CONTENTS CONTENTS (Continued) Introduction ............................................................................................................1 Appendix A: Summary Chart of U.S. Medical Eligibility Criteria for Methods ....................................................................................................................2 Contraceptive Use, 2010 .................................................................................. 47 How To Use This Document ...............................................................................3 Appendix B: When To Start Using Specific Contraceptive Summary of Changes from WHO SPR ............................................................4 Methods .............................................................................................................. 55 Contraceptive Method Choice .........................................................................4 Appendix C: Examinations and Tests Needed Before Initiation of Maintaining Updated Guidance ......................................................................4 Contraceptive Methods -
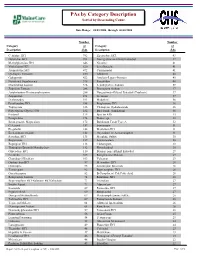
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Printed Formulary Catalog Basic
Scripps Health Formulary July 2016 Foreword Pharmacy and Therapeutics Committee. MedImpact approves such multi- This document represents the efforts of the MedImpact Healthcare Systems source drugs for addition to the MAC list based on the following criteria: Pharmacy and Therapeutics (P & T) and Formulary Committees to provide physicians A multi-source drug product manufactured by at least one (1) nationally and pharmacists with a method to evaluate the safety, efficacy and cost-effectiveness marketed company. of commercially available drug products. A structured approach to the drug selection At least one (1) of the generic manufacturer’s products must have an “A” process is essential in ensuring continuing patient access to rational drug therapies. rating or the generic product has been determined to be unassociated with The ultimate goal of the Portfolio Formulary is to provide a process and framework to efficacy, safety or bioequivalency concerns by the MedImpact P & T support the dynamic evolution of this document to guide prescribing decisions that Committee. reflect the most current clinical consensus associated with drug therapy decisions. Drug product will be approved for generic substitution by the MedImpact P & T Committee. This is accomplished through the auspices of the MedImpact P & T and Formulary Committees. These committees meet quarterly and more often as warranted to ensure This list is reviewed and updated periodically based on the clinical literature and clinical relevancy of the Formulary. To accommodate changes to this document, pharmacokinetic characteristics of currently available versions of these drug updates are made accessible as necessary. products. As you use this Formulary, you are encouraged to review the information and provide If a member or physician requests a brand name product in lieu of an approved your input and comments to the MedImpact P & T and Formulary Committees. -

USP Reference Standards Catalog
Last Updated On: January 6, 2016 USP Reference Standards Catalog Catalog # Description Current Lot Previous Lot CAS # NDC # Unit Price Special Restriction 1000408 Abacavir Sulfate R028L0 F1L487 (12/16) 188062-50-2 $222.00 (200 mg) 1000419 Abacavir Sulfate F0G248 188062-50-2 $692.00 Racemic (20 mg) (4-[2-amino-6-(cyclo propylamino)-9H-pur in-9yl]-2-cyclopenten e-1-methanol sulfate (2:1)) 1000420 Abacavir Related F1L311 F0H284 (10/13) 124752-25-6 $692.00 Compound A (20 mg) ([4-(2,6-diamino-9H- purin-9-yl)cyclopent- 2-enyl]methanol) 1000437 Abacavir Related F0M143 N/A $692.00 Compound D (20 mg) (N6-Cyclopropyl-9-{( 1R,4S)-4-[(2,5-diami no-6-chlorpyrimidin- 4-yloxy)methyl] cyclopent-2-enyl}-9H -purine-2,6-diamine) 1000441 Abacavir Related F1L318 F0H283 (10/13) N/A $692.00 Compound B (20 mg) ([4-(2,5-diamino-6-c Page 1 Last Updated On: January 6, 2016 USP Reference Standards Catalog Catalog # Description Current Lot Previous Lot CAS # NDC # Unit Price Special Restriction hloropyrimidin-4-yla mino)cyclopent-2-en yl]methanol) 1000452 Abacavir Related F1L322 F0H285 (09/13) 172015-79-1 $692.00 Compound C (20 mg) ([(1S,4R)-4-(2-amino -6-chloro-9H-purin-9 -yl)cyclopent-2-enyl] methanol hydrochloride) 1000485 Abacavir Related R039P0 F0J094 (11/16) N/A $692.00 Compounds Mixture (15 mg) 1000496 Abacavir F0J102 N/A $692.00 Stereoisomers Mixture (15 mg) 1000500 Abacavir System F0J097 N/A $692.00 Suitability Mixture (15 mg) 1000521 Acarbose (200 mg) F0M160 56180-94-0 $222.00 (COLD SHIPMENT REQUIRED) 1000532 Acarbose System F0L204 N/A $692.00 Suitability -

Pharmacy Program and Drug Formulary
Pharmacy Program and Drug Formulary Secure Horizons Group Retiree Medicare Advantage Plan n Pharmacy Program Description n Platinum Plus Enhanced Formulary California Benefits Effective January 1, 2006 Table of Contents Your Secure Horizons Group Retiree Medicare Advantage Plan Prescription Drug Benefit........................................................................................................ iii Secure Horizons Pharmacy Program Definitions .................................................................... iii What Is the Platinum Plus Enhanced Formulary? ....................................................................iv Where to Have Your Prescriptions Filled .................................................................................iv Preferred and Non-Preferred Network Pharmacies .................................................................iv Network Preferred Pharmacy Locations ..................................................................................iv How to Fill a Prescription at a Network Pharmacy ...................................................................v Mail Service Pharmacy ..............................................................................................................v Secure Horizons Group Retiree Medicare Advantage Plan Offers a Two-Part Prescription Drug Benefit ..........................................................................vii Part 1 – Medicare Part D Prescription Drug Coverage ...................................................vii How Your Medicare -

Norgestimate and Ethinyl Estradiol Tablets USP, 0.25 Mg/0.035 Mg
NORGESTIMATE AND ETHINYL ESTRADIOL- norgestimate and ethinyl estradiol Glenmark Generics Inc., USA ---------- Norgestimate and Ethinyl Estradiol Tablets USP, 0.25 mg/0.035 mg Patients should be counseled that this product does not protect against HIV infection (AIDS) and other sexually transmitted diseases. DESCRIPTION The following product is a combination oral contraceptive containing the progestational compound norgestimate USP and the estrogenic compound ethinyl estradiol USP. Norgestimate and ethinyl estradiol tablets USP: Each blue tablet contains 0.25 mg of the progestational compound, norgestimate USP (18,19-Dinor-17- pregn-4-en-20-yn-3-one,17-(acetyloxy)-13-ethyl-,oxime,(17α)-(+)-) and 0.035 mg of the estrogenic compound, ethinyl estradiol USP (19-nor-17α-pregna,1,3,5(10)-trien-20-yne-3,17-diol). Inactive ingredients include lactose monohydrate, povidone, FD&C Blue No. 2-5625, colloidal silicon dioxide, talc, magnesium stearate, and pregelatinized starch. Each light green tablet contains only inert ingredients, as follows: D&C Yellow No. 10, FD&C Blue No. 2-5625, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinized starch and talc. Norgestimate USP Ethinyl Estradiol USP CLINICAL PHARMACOLOGY Oral Contraception Combination oral contraceptives act by suppression of gonadotropins. Although the primary mechanism of this action is inhibition of ovulation, other alterations include changes in the cervical mucus (which increase the difficulty of sperm entry into the uterus) and the endometrium (which reduce the likelihood of implantation). Receptor binding studies, as well as studies in animals and humans, have shown that norgestimate and 17-deacetyl norgestimate, the major serum metabolite, combine high progestational activity with minimal intrinsic androgenicity 90-93. -

Other Data Relevant to an Evaluation of Carcinogenicity and Its Mechanisms
COMBINED ESTROGEN−PROGESTOGEN CONTRACEPTIVES 143 4. Other Data Relevant to an Evaluation of Carcinogenicity and its Mechanisms 4.1 Absorption, distribution, metabolism and excretion in humans The metabolism and disposition of various formulations of oral contraceptives used in humans differ. After entering the small intestine, estrogenic and progestogenic compounds in combined oral contraceptives undergo metabolism by bacterial enzymes and enzymes in the intestinal mucosa to varying extents. The mixture of metabolized and unmetabolized compounds then undergoes intestinal absorption, and thus enters the portal vein blood, which perfuses the liver. In the liver, the compounds can be metabolized extensively, which leads to variations in the amount of active drug. A fraction of the absorbed dose of ethinyl- estradiol and some progestogens is also excreted in the bile during its first transit through the liver. Although some of these compounds are partially reabsorbed via the enterohepatic circulation, a fraction may also be excreted in this ‘first pass’, which reduces overall bio- availability. Since steroids penetrate normal skin easily, various systems have also been developed that deliver estrogens and progestogens parenterally, e.g. transdermal patches, nasal sprays, subcutaneous implants, vaginal rings and intrauterine devices (Fanchin et al., 1997; Dezarnaulds & Fraser, 2002; Meirik et al., 2003; Sarkar, 2003; Wildemeersch et al., 2003; Sturdee et al., 2004). These different modes of administration have been described previously (IARC, 1999). In general, all parenteral routes avoid loss of the drug by hepatic first-pass metabolism and minimally affect hepatic protein metabolism. The absorption rates of orally administered estrogens and progestogens are usually rapid; peak serum values are observed between 0.5 and 4 h after intake. -

Pairwise Comparisons
National Institute for Health and Care Excellence Final Acne vulgaris: management [E2] Management options for mild to moderate acne – pairwise comparisons NG198 Evidence review underpinning recommendations 1.5.1, 1.5.2 and 1.5.5 to 1.5.14 (excluding 1.5.6 which is underpinned by evidence review L, 1.5.10 and bullet points 2 and 3 of recommendation 1.5.12 which are underpinned by evidence review F1 and 3 research recommendations in the NICE guideline - see evidence review E1 for the committee’s discussion of the evidence) June 2021 Final These evidence reviews were developed by the National Guideline Alliance which is a part of the Royal College of Obstetricians and Gynaecologists FINAL Error! No text of specified style in document. Disclaimer The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian. Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities.