Contraception and Misconceptions
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Integrating Complementary Medicine Into Cardiovascular Medicine
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Journal of the American College of Cardiology Vol. 46, No. 1, 2005 © 2005 by the American College of Cardiology Foundation ISSN 0735-1097/05/$30.00 Published by Elsevier Inc. doi:10.1016/j.jacc.2005.05.031 ACCF COMPLEMENTARY MEDICINE EXPERT CONSENSUS DOCUMENT Integrating Complementary Medicine Into Cardiovascular Medicine A Report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (Writing Committee to Develop an Expert Consensus Document on Complementary and Integrative Medicine) WRITING COMMITTEE MEMBERS JOHN H. K. VOGEL, MD, MACC, Chair STEVEN F. BOLLING, MD, FACC BRIAN OLSHANSKY, MD, FACC REBECCA B. COSTELLO, PHD KENNETH R. PELLETIER, MD(HC), PHD ERMINIA M. GUARNERI, MD, FACC CYNTHIA M. TRACY, MD, FACC MITCHELL W. KRUCOFF, MD, FACC, FCCP ROBERT A. VOGEL, MD, FACC JOHN C. LONGHURST, MD, PHD, FACC TASK FORCE MEMBERS ROBERT A. VOGEL, MD, FACC, Chair JONATHAN ABRAMS, MD, FACC SANJIV KAUL, MBBS, FACC JEFFREY L. ANDERSON, MD, FACC ROBERT C. LICHTENBERG, MD, FACC ERIC R. BATES, MD, FACC JONATHAN R. LINDNER, MD, FACC BRUCE R. BRODIE, MD, FACC* ROBERT A. O’ROURKE, MD, FACC† CINDY L. GRINES, MD, FACC GERALD M. POHOST, MD, FACC PETER G. DANIAS, MD, PHD, FACC* RICHARD S. SCHOFIELD, MD, FACC GABRIEL GREGORATOS, MD, FACC* SAMUEL J. SHUBROOKS, MD, FACC MARK A. HLATKY, MD, FACC CYNTHIA M. TRACY, MD, FACC* JUDITH S. HOCHMAN, MD, FACC* WILLIAM L. WINTERS, JR, MD, MACC* *Former members of Task Force; †Former chair of Task Force The recommendations set forth in this report are those of the Writing Committee and do not necessarily reflect the official position of the American College of Cardiology Foundation. -

The National Drugs List
^ ^ ^ ^ ^[ ^ The National Drugs List Of Syrian Arab Republic Sexth Edition 2006 ! " # "$ % &'() " # * +$, -. / & 0 /+12 3 4" 5 "$ . "$ 67"5,) 0 " /! !2 4? @ % 88 9 3: " # "$ ;+<=2 – G# H H2 I) – 6( – 65 : A B C "5 : , D )* . J!* HK"3 H"$ T ) 4 B K<) +$ LMA N O 3 4P<B &Q / RS ) H< C4VH /430 / 1988 V W* < C A GQ ") 4V / 1000 / C4VH /820 / 2001 V XX K<# C ,V /500 / 1992 V "!X V /946 / 2004 V Z < C V /914 / 2003 V ) < ] +$, [2 / ,) @# @ S%Q2 J"= [ &<\ @ +$ LMA 1 O \ . S X '( ^ & M_ `AB @ &' 3 4" + @ V= 4 )\ " : N " # "$ 6 ) G" 3Q + a C G /<"B d3: C K7 e , fM 4 Q b"$ " < $\ c"7: 5) G . HHH3Q J # Hg ' V"h 6< G* H5 !" # $%" & $' ,* ( )* + 2 ا اوا ادو +% 5 j 2 i1 6 B J' 6<X " 6"[ i2 "$ "< * i3 10 6 i4 11 6! ^ i5 13 6<X "!# * i6 15 7 G!, 6 - k 24"$d dl ?K V *4V h 63[46 ' i8 19 Adl 20 "( 2 i9 20 G Q) 6 i10 20 a 6 m[, 6 i11 21 ?K V $n i12 21 "% * i13 23 b+ 6 i14 23 oe C * i15 24 !, 2 6\ i16 25 C V pq * i17 26 ( S 6) 1, ++ &"r i19 3 +% 27 G 6 ""% i19 28 ^ Ks 2 i20 31 % Ks 2 i21 32 s * i22 35 " " * i23 37 "$ * i24 38 6" i25 39 V t h Gu* v!* 2 i26 39 ( 2 i27 40 B w< Ks 2 i28 40 d C &"r i29 42 "' 6 i30 42 " * i31 42 ":< * i32 5 ./ 0" -33 4 : ANAESTHETICS $ 1 2 -1 :GENERAL ANAESTHETICS AND OXYGEN 4 $1 2 2- ATRACURIUM BESYLATE DROPERIDOL ETHER FENTANYL HALOTHANE ISOFLURANE KETAMINE HCL NITROUS OXIDE OXYGEN PROPOFOL REMIFENTANIL SEVOFLURANE SUFENTANIL THIOPENTAL :LOCAL ANAESTHETICS !67$1 2 -5 AMYLEINE HCL=AMYLOCAINE ARTICAINE BENZOCAINE BUPIVACAINE CINCHOCAINE LIDOCAINE MEPIVACAINE OXETHAZAINE PRAMOXINE PRILOCAINE PREOPERATIVE MEDICATION & SEDATION FOR 9*: ;< " 2 -8 : : SHORT -TERM PROCEDURES ATROPINE DIAZEPAM INJ. -

Investigation of Influence of Diazepam, Valproate, Cyproheptadine and Cortisol on the Rewarding Ventral Tegmental Self-Stimulation Behaviour
Ind. J. Physio!. Pharmac., Volume 33, Number 3, 1989 INVESTIGATION OF INFLUENCE OF DIAZEPAM, VALPROATE, CYPROHEPTADINE AND CORTISOL ON THE REWARDING VENTRAL TEGMENTAL SELF-STIMULATION BEHAVIOUR SONTI V. RAMANA AND T. DESIRAJU· Department ofNeurophysiology, National Institute of Mental Health arzd Neuro Sciences, Bangalore - 560 029 ( Received on June IS, 1989 ) Abstract: Experiments were carried on in the Wistar rats having self-stimulation (88) electrodes implanted chronically in substantia nigra-ventral tegmental area (SN-VTA) to examine whether modulations of GABAergic, serotonergic, histaminergic, dopaminergic, and glucocorticoid neuronal receptor functions will affect or not the brain reward system and the 88 behaviour. The modulalon are the wellknown drugs: diazepam which is a facilitator ohome of the GABA recepton, and used clinically (or its tranquilising, anxiolytic, sedative-hypnotic and anti-convulsant properties: sodium valproate which is known to enhance the GABA synapse function, and used clinically for its anti convulsant property; haloperidol which is a dopaminergic receptor (D2) blocker, and clinically used for its anti-psychotic property; cyproheptadine which is both anti-histaminic and anti-serotonergic (blocka 5-HT2 receptor), used clinically for its antihistaminic and other beneficial properties; and hydrocortisone which is the stress-resisting glucocorticoid having direct effects on both brain and body cells, used clinically for the wide-ranging glucocorticoid therapeutic effects. The results revealed that systemic administration of these drugs, except haloperidol, caused no significant influenee on the 88 behaviour, thereby indicating that these nondopaminergic drugs have no effect on brain-reward system and also these categories of synaptic actions are not likely to be involved in the primary organization of the mechanisms of the brain-reward system. -

1493 JP XV Infrared Reference Spectra
JP XV Infrared Reference Spectra 1493 Roxatidine Acetate Hydrochloride Preparation of sample: Potassium chloride disk method Roxithromycin Preparation of sample: Potassium bromide disk method Saccharin Preparation of sample: Potassium bromide disk method 1494 Infrared Reference Spectra JP XV Saccharin Sodium Hydrate Preparation of sample: Potassium bromide disk method Salbutamol Sulfate Preparation of sample: Potassium bromide disk method Santonin Preparation of sample: Potassium bromide disk method JP XV Infrared Reference Spectra 1495 Scopolamine Butylbromide Preparation of sample: Potassium bromide disk method Siccanin Preparation of sample: Potassium bromide disk method Sodium Fusidate Preparation of sample: Potassium bromide disk method 1496 Infrared Reference Spectra JP XV Sodium Picosulfate Hydrate Preparation of sample: Potassium bromide disk method Sodium Polystyrene Sulfonate Preparation of sample: Potassium bromide disk method Sodium Prasterone Sulfate Hydrate Preparation of sample: Potassium bromide disk method JP XV Infrared Reference Spectra 1497 Sodium Salicylate Preparation of sample: Potassium bromide disk method Sodium Valproate Preparation of sample: Liquid film method Spiramycin Acetate Preparation of sample: Potassium bromide disk method 1498 Infrared Reference Spectra JP XV Spironolactone Preparation of sample: Potassium bromide disk method Sulbactam Sodium Preparation of sample: Potassium bromide disk method Sulbenicillin Sodium Preparation of sample: Potassium bromide disk method JP XV Infrared Reference Spectra -

Effect of Picrotoxin and Cyproheptadine Pretreatment On
Int J Biol Med Res. 2012; 3(2): 1606-1608 Int J Biol Med Res www.biomedscidirect.com Volume 2, Issue 4, Jan 2012 Contents lists available at BioMedSciDirect Publications International Journal of Biological & Medical Research BioMedSciDirect Journal homepage: www.biomedscidirect.com International Journal of Publications BIOLOGICAL AND MEDICAL RESEARCH Original article Effect of picrotoxin and cyproheptadine pretreatment on sodium valproate induced wet dog shake behavior in rats B M Sattigeri*, J J Balsara, J H Jadhav Department of pharmacology, Sumandeep Vidyapeeth’s SBKS & MIRC, Piparia, Vadodra Gujaart Department of pharmacology, Krishna Institute of Medical Sciences, Malkapur, Karad (Dist.Satara) 415110, Maharashtra, India A R T I C L E I N F O A B S T R A C T Keywords: Sodium valproate, a broad spectrum antiepileptic elevates the brain GABA levels by various Cyproheptadine mechanisms. Histological, electrophysiological and the biochemical studies suggest a Picrotoxin Sodium valproate regulatory role of GABA on dopaminergic neurons. Behavioral studies in animals provide an Wet Dog Shake Behavior additional evidence for interaction between GABAergic and DAergic systems. Valproate at 200- 500mg/kg induces Wet Dog Shake (WDS) behavior in rats. The WDS behavior in rats and head twitch responses in mice is evoked by 5- hydroxy-tryptophan (5-HT), the 5-HT precursor, the directly acting non-selective 5-HT receptor agonists and 5-HT releasers. In order to determine the involvement of GABAergic and 5-HTergic mechanism in the induction of WDS behavior by valproate in rats, the study was taken upto investigate the effect picrotoxin and cyproheptadine pretreatment on valproate induced WDS in rats. -

209627Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 209627Orig1s000 MULTI-DISCIPLINE REVIEW Summary Review Office Director Cross Discipline Team Leader Review Clinical Review Non-Clinical Review Statistical Review Clinical Pharmacology Review Reviewers of Multi-Disciplinary Review and Evaluation SECTIONS OFFICE/ AUTHORED/ ACKNOWLEDGED/ DISCIPLINE REVIEWER DIVISION APPROVED Mark Seggel, Ph.D. OPQ/ONDP/DNDP2 Authored: Section 4.2 Digitally signed by Mark R. Seggel -S CMC Lead DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, cn=Mark R. Signature: Mark R. Seggel -S Seggel -S, 0.9.2342.19200300.100.1.1=1300071539 Date: 2018.08.08 16:29:15 -04'00' Frederic Moulin, DVM, PhD OND/ODE3/DBRUP Authored: Section 5 Pharmacology/ Digitally signed by Frederic Moulin -S Toxicology DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Reviewer Signature: Frederic Moulin -S 0.9.2342.19200300.100.1.1=2001708658, cn=Frederic Moulin -S Date: 2018.08.08 15:26:57 -04'00' Kimberly Hatfield, PhD OND/ODE3/DBRUP Approved: Section 5 Pharmacology/ Toxicology Digitally signed by Kimberly P. Hatfield -S DN: c=US, o=U.S. Government, ou=HHS, ou=FDA, ou=People, Team Leader Signature: Kimberly P. Hatfield -S 0.9.2342.19200300.100.1.1=1300387215, cn=Kimberly P. Hatfield -S Date: 2018.08.08 14:56:10 -04'00' Li Li, Ph.D. OCP/DCP3 Authored: Sections 6 and 17.3 Clinical Pharmacology Dig ta ly signed by Li Li S DN c=US o=U S Government ou=HHS ou=FDA ou=People Reviewer cn=Li Li S Signature: Li Li -S 0 9 2342 19200300 100 1 1=20005 08577 Date 2018 08 08 15 39 23 04'00' Doanh Tran, Ph.D. -

Estrogen and Progestin Hormone Doses in Combined Birth Control Pills
Estrogen and Progestin Hormone Doses in Combined Birth Control Pills Estrogen level Pill Brand Name Progestin Dose (mg) ethinyl estradiol (micrograms) 20 mcgm Alesse® levonorgestrel 0.10 Levlite® levonorgestrel 0.10 Loestrin 1/20® Fe norethindrone 1.00 acetate Mircette® desogestrel 0.15 Ortho Evra® norelgestromin 0.15 (patch) (norgestimate metabolite) phasic Estrostep® Fe norethindrone 1.0/1.0/1.0 20/30/35 mcgm acetate 30 mcgm Levlen® levonorgestrel 0.15 Levora® levonorgestrel 0.15 Nordette® levonorgestrel 0.15 Lo/Ovral® norgestrel 0.30 Desogen® desogestrel 0.15 Ortho-Cept® desogestrel 0.15 Loestrin® 1.5/30 norethindrone 1.50 acetate Yasmin® drospirenone 3.0 phasic Triphasil® levonorgestrel 0.05/0.075/0.125 30/40/30 mcgm Tri-Levlen® levonorgestrel 0.05/0.075/0.125 Trivora® levonorgestrel 0.05/0.075/0.125 35 mcgm Ortho-Cyclen® norgestimate 0.25 Ovcon-35® norethindrone 0.40 Brevicon® norethindrone 0.50 Modicon® norethindrone 0.50 Necon® norethindrone 1.00 Norethin® norethindrone 1.00 Norinyl® 1/35 norethindrone 1.00 Ortho-Novum® 1/35 norethindrone 1.00 Demulen® 1/35 ethynodiol diacetate 1.00 Zovia® 1/35E ethynodiol diacetate 1.00 phasic Ortho-Novum® norethindrone 0.50/1.00 35/35 mcgm 10/11 Jenest® norethindrone 0.50/1.00 phasic Ortho-Tri-Cyclen® norgestimate 0.15/0.215/0.25 35/35/35 mcgm Ortho-Novum® norethindrone 0.50/0.75/1.00 7/7/7 Tri-Norinyl® norethindrone 0.50/1.00/0.50 50 mcgm Necon® 1/50 norethindrone 1.00 Norinyl® 1/50 norethindrone 1.00 Ortho-Novum® 1/50 norethindrone 1.00 Ovcon-50® norethindrone 1.00 Ovral® norgestrel 0.50 Demulen® 1/50 ethynodiol diacetate 1.00 Zovia® 1/50E ethynodiol diacetate 1.00 Which pills have higher progestin side efects or cause more acne and hair growth? Each progestin has a diferent potency, milligram per milligram, in terms of progesterone efect to stop menstrual bleeding or androgen efect to stimulate acne and hair growth. -

Valproate-Olanzapine-Nifedipine Interaction Related Pedal Edema
Letter-to-the Editor Taiwanese Journal of Psychiatry (Taipei) Vol. 28 No. 3 2014 • 181 • Valproate-olanzapine-nifedipine Interaction Related Pedal Edema Patient’s bilateral pedal pitting edema did not Case Report resolve spontaneously after discontinuing olan- zapine until he received diuretics with furosemide A 45-year-old single male patient with bipo- 40 mg/day and spironolactone 75 mg/day on the lar I disorder was admitted to acute ward of our 21st day of pitting edema. He required further hospital due to depressive mood with irritability treatment, and had normal fi ndings in physical ex- for more than one month. The fi nding of physical amination and repeated laboratory tests. examination at admission revealed no bilateral pedal edema under long-term usage of valproate Comment 700 mg/day as mood stabilizer, nifedipine 30 mg/ day for essential hypertension, and allopurinol There have been some clinical reports to as- 100 mg/day for gouty arthritis before hospitaliza- sociate olanzapine treatment with bilateral lower tion. The results of laboratory tests for electro- extremity edema since 2000 [1]. Pre-clinical trials lytes, renal function, liver function, chest X-ray, of oral olanzapine (doses being equal or greater and electrocardiography were also within normal than 2.5 mg/day) have also reported peripheral limits. edema as a rare adverse event. The pathophysio- During this hospitalization, we added olan- logical mechanism of low extremity edema re- zapine 5 mg/day to control manic symptoms. Five mains unknown, although some mechanisms days later, he reported bilateral swelling over low- could be proposed, such as olanzapine’s blockage er legs and ankles, a bilateral 2+ pitting edema in of α1, M1, M3 and 5-HT2 receptors [2, 3]. -

U.S. Medical Eligibility Criteria for Contraceptive Use, 2016
Morbidity and Mortality Weekly Report Recommendations and Reports / Vol. 65 / No. 3 July 29, 2016 U.S. Medical Eligibility Criteria for Contraceptive Use, 2016 U.S. Department of Health and Human Services Centers for Disease Control and Prevention Recommendations and Reports CONTENTS Introduction ............................................................................................................1 Methods ....................................................................................................................2 How to Use This Document ...............................................................................3 Keeping Guidance Up to Date ..........................................................................5 References ................................................................................................................8 Abbreviations and Acronyms ............................................................................9 Appendix A: Summary of Changes from U.S. Medical Eligibility Criteria for Contraceptive Use, 2010 ...........................................................................10 Appendix B: Classifications for Intrauterine Devices ............................. 18 Appendix C: Classifications for Progestin-Only Contraceptives ........ 35 Appendix D: Classifications for Combined Hormonal Contraceptives .... 55 Appendix E: Classifications for Barrier Methods ..................................... 81 Appendix F: Classifications for Fertility Awareness–Based Methods ..... 88 Appendix G: Lactational -

Recommendations for Contraceptive Use, 2013 Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2Nd Edition
Morbidity and Mortality Weekly Report Early Release / Vol. 62 June 14, 2013 U.S. Selected Practice Recommendations for Contraceptive Use, 2013 Adapted from the World Health Organization Selected Practice Recommendations for Contraceptive Use, 2nd Edition Continuing Education Examination available at http://www.cdc.gov/mmwr/cme/conted.html. U.S. Department of Health and Human Services Centers for Disease Control and Prevention Early Release CONTENTS CONTENTS (Continued) Introduction ............................................................................................................1 Appendix A: Summary Chart of U.S. Medical Eligibility Criteria for Methods ....................................................................................................................2 Contraceptive Use, 2010 .................................................................................. 47 How To Use This Document ...............................................................................3 Appendix B: When To Start Using Specific Contraceptive Summary of Changes from WHO SPR ............................................................4 Methods .............................................................................................................. 55 Contraceptive Method Choice .........................................................................4 Appendix C: Examinations and Tests Needed Before Initiation of Maintaining Updated Guidance ......................................................................4 Contraceptive Methods -
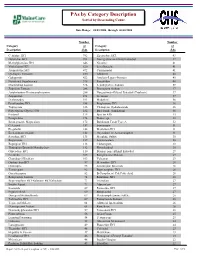
Report 752 by Category Description
PAs by Category Description Sorted by Descending Count Date Range: 04/01/2006 through 06/30/2006 Number Number Category of Category of Description PAs Description PAs Cetirizine HCl 792 Ziprasidone HCl 43 Duloxetine HCl 784 Norelgestromin-Ethinyl Estradiol 42 Methylphenidate HCl 646 Nicotine 41 Venlafaxine HCl 620 Levofloxacin 41 Atomoxetine HCl 472 Carisoprodol 41 Quetiapine Fumarate 430 Albuterol 40 Gabapentin 422 Amylase-Lipase-Protease 40 Nutritional Supplements 378 Famotidine 40 Montelukast Sodium 326 Levothyroxine Sodium 39 Zolpidem Tartrate 288 Enoxaparin Sodium 37 Amphetamine-Dextroamphetamine 286 Norgestimate-Ethinyl Estradiol (Triphasic) 37 Aripiprazole 271 Tretinoin 37 Desloratadine 191 Modafinil 36 Fexofenadine HCl 188 Pioglitazone HCl 36 Topiramate 186 Citalopram Hydrobromide 36 Polyethylene Glycol 3350 182 Budesonide (Inhalation) 36 Fentanyl 175 Epoetin Alfa 33 Eszopiclone 174 Etanercept 33 Esomeprazole Magnesium 172 Botulinum Toxin Type A 32 Celecoxib 157 Somatropin 31 Pregabalin 148 Metformin HCl 31 Escitalopram Oxalate 142 Oxycodone w/ Acetaminophen 31 Sertraline HCl 135 Morphine Sulfate 30 Risperidone 127 Levetiracetam 30 Bupropion HCl 118 Clonazepam 30 Tiotropium Bromide Monohydrate 112 Phenobarbital 30 Oxycodone HCl 110 Drospirenone-Ethinyl Estradiol 29 Ezetimibe 105 Rosiglitazone Maleate 29 Clopidogrel Bisulfate 103 Valsartan 29 Ondansetron HCl 97 Memantine HCl 28 Olanzapine 93 Sumatriptan Succinate 28 Temazepam 92 Buprenorphine HCl 28 Oxcarbazepine 82 B-Complex w/ C & Folic Acid 28 Rabeprazole Sodium 74 Ranitidine -

Printed Formulary Catalog Basic
Scripps Health Formulary July 2016 Foreword Pharmacy and Therapeutics Committee. MedImpact approves such multi- This document represents the efforts of the MedImpact Healthcare Systems source drugs for addition to the MAC list based on the following criteria: Pharmacy and Therapeutics (P & T) and Formulary Committees to provide physicians A multi-source drug product manufactured by at least one (1) nationally and pharmacists with a method to evaluate the safety, efficacy and cost-effectiveness marketed company. of commercially available drug products. A structured approach to the drug selection At least one (1) of the generic manufacturer’s products must have an “A” process is essential in ensuring continuing patient access to rational drug therapies. rating or the generic product has been determined to be unassociated with The ultimate goal of the Portfolio Formulary is to provide a process and framework to efficacy, safety or bioequivalency concerns by the MedImpact P & T support the dynamic evolution of this document to guide prescribing decisions that Committee. reflect the most current clinical consensus associated with drug therapy decisions. Drug product will be approved for generic substitution by the MedImpact P & T Committee. This is accomplished through the auspices of the MedImpact P & T and Formulary Committees. These committees meet quarterly and more often as warranted to ensure This list is reviewed and updated periodically based on the clinical literature and clinical relevancy of the Formulary. To accommodate changes to this document, pharmacokinetic characteristics of currently available versions of these drug updates are made accessible as necessary. products. As you use this Formulary, you are encouraged to review the information and provide If a member or physician requests a brand name product in lieu of an approved your input and comments to the MedImpact P & T and Formulary Committees.