From Shoot to Leaf: Step-Wise Shifts in Meristem and KNOX1 Activity Correlate with the Evolution of a Unifoliate Body Plan in Gesneriaceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Newsletter 123 May 2012
TheTheThe Irish Garden Plant Society Newsletter Number 11123123 May 2012 The Annual General Meeting 2012 The Annual General Meeting will be held on Sat 12th May 10.00 a.m for 10.30 a.m., at Hillsborough Courthouse, The Square, Hillsborough, BT26 6AG. As always, it will be followed by a series of garden visits on Saturday & Sunday and a meal on Saturday evening. The meal will be held at 8:00pm in La Mon Hotel & Country Club, 41 Gransha Road, Comber, BT23 5RF. See the January 2012 newsletter for details of the gardens to be visited. If you haven’t already booked contact Patrick Quigley, 24 Areema Drive, Dunmurry, Belfast, BT17 0QG. tel: +44 (0) 7801 299263 [email protected] for further information. A.G. M. Agenda 1. Apologies 2. Minutes of AGM 2011 3. Matters arising 4. Chairman’s report 5. Treasurer’s report 6. Election of Committee Members 7. Any other business Front cover : Solanum crispum ‘Glasnevin’. Photograph : Pearse Rowe In this issue 2 Editorial 3 Southern Climbers for Northern Walls by John Joe Costin 11 Worth a Read by Paddy Tobin 14 The Palm House, a review by Mary Bradshaw 16 Cheers to Chiltern Seeds and to the Sole Survivor of Seed Project 1997/98 by Michael Kelleher 17 Seed Distribution Report 2011 and 2012 by Stephen Butler 19 Regional Reports 28 Spring at Kilmacurragh by Seamus O’Brien 37 Propagation of Arbutus by Kevin Line 41 Tulipa ‘Molly Bloom’ - an new Tulip for 2012 1 Editorial Spring time and new plants are synonymous, and as Christopher Lloyd said in Garden Flowers from Seed, seed sowing is “one of life’s big thrills”. -

Evolution of Unusual Morphologies in Lentibulariaceae (Bladderworts and Allies) And
Annals of Botany 117: 811–832, 2016 doi:10.1093/aob/mcv172, available online at www.aob.oxfordjournals.org REVIEW: PART OF A SPECIAL ISSUE ON DEVELOPMENTAL ROBUSTNESS AND SPECIES DIVERSITY Evolution of unusual morphologies in Lentibulariaceae (bladderworts and allies) and Podostemaceae (river-weeds): a pictorial report at the interface of developmental biology and morphological diversification Rolf Rutishauser* Institute of Systematic Botany, University of Zurich, Zurich, Switzerland * For correspondence. E-mail [email protected] Received: 30 July 2015 Returned for revision: 19 August 2015 Accepted: 25 September 2015 Published electronically: 20 November 2015 Background Various groups of flowering plants reveal profound (‘saltational’) changes of their bauplans (archi- tectural rules) as compared with related taxa. These plants are known as morphological misfits that appear as rather Downloaded from large morphological deviations from the norm. Some of them emerged as morphological key innovations (perhaps ‘hopeful monsters’) that gave rise to new evolutionary lines of organisms, based on (major) genetic changes. Scope This pictorial report places emphasis on released bauplans as typical for bladderworts (Utricularia,approx. 230 secies, Lentibulariaceae) and river-weeds (Podostemaceae, three subfamilies, approx. 54 genera, approx. 310 species). Bladderworts (Utricularia) are carnivorous, possessing sucking traps. They live as submerged aquatics (except for their flowers), as humid terrestrials or as epiphytes. Most Podostemaceae are restricted to rocks in tropi- http://aob.oxfordjournals.org/ cal river-rapids and waterfalls. They survive as submerged haptophytes in these extreme habitats during the rainy season, emerging with their flowers afterwards. The recent scientific progress in developmental biology and evolu- tionary history of both Lentibulariaceae and Podostemaceae is summarized. -
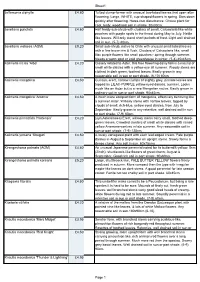
Sheet1 Jeffersonia Diphylla £4.80 Tufted Clump-Former with Unusual Two-Lobed Leaves That Open After Flowering
Sheet1 Jeffersonia diphylla £4.80 Tufted clump-former with unusual two-lobed leaves that open after flowering. Large, WHITE, cup-shaped flowers in spring. Dies down quickly after flowering. Hates root disturbance. Choice plant for cool, leafy, woodland soil in shade. 30x30cm. Jovellana punctata £4.60 Half hardy sub-shrub with clusters of small, Calceolaria like white pouches with purple spots in the throat during May to July. Nettle like leaves. Will only stand short periods of frost. Light well drained soil in sun. (5-7) 40cm. Jovellana violacea (AGM) £8.20 Small sub-shrub, native to Chile with unusual small lobed leaves with a fine brown rim & flush. Clusters of Calceolaria like, small lilac purple flowers like small pouches;- spring through summer. Needs a warm spot or cold greenhouse in winter. (5-8) 45x45cm. Kalimeris incisa 'Alba' £4.20 Closely related to Aster, this free flowering daisy forms a mound of small white daisies with a yellow eye all summer. Woody stems are clothed in dark green, toothed leaves. Easily grown in any reasonable soil in sun or part shade. (6-10) 60cm Kalimeris mongolica £4.50 Curious, erect, narrow clumps of slightly grey, pinnate leaves are topped by LILAC-PURPLE yellow-eyed daisies; summer. Looks much like an Aster but is a rare Mongolian native. Easily grown in ordinary soil in sun or part shade. 90x45cm. Kalimeris mongolica 'Antonia' £4.50 A much more compact form of mongolica, effectively behaving like a summer Aster. Willowy stems with narrow leaves, topped by clouds of small, rich-blue, yellow eyed daisies, from July to September. -

Show Schedules 2012 Ver Finale
119. 1 pan rock plant native to the Southern Hemisphere 120. 1 pan dwarf shurb THE SCOTTISH ROCK GARDEN CLUB 121. 1 pan rock plant raised from seed by the exhibitor. Date of sowing to be stated. Botanical notes permitted, AGS note 23(e) SECTION III Open to Amateur Members of AGS and SRGC who have not won an AGS Bronze Merit Medal or more than ten First Prizes at Shows run by either Society prior to 1st January 2011. Pan size not to exceed 19 cm outside diameter 130. 3 pans rock plants, distinct 131. 1 pan rock plant in flower 132. 1 pan Gentiana 133. 1 pan Cyclamen 134. 1 pan bulbous plant 135. 1 pan rock plant native to the Southern Hemisphere 136. 1 pan rock plant native to the Northern Hemisphere 137. 1 pan rock plant for foliage effect 138. 1 pan dwarf shrub or conifer 139. 1 pan rock plant. For exhibitors who have never won a first prize at an AGS or SRGC National show SHOW SCHEDULES 2012 DUNBLANE EARLY BULB DISPLAY 18th February* BLACKPOOL SHOW 17th March* STIRLING SHOW 24th March† New Location - Show this Year is in KINCARDINE NORTHUMBERLAND 40th ANNIVERSARY SHOW, HEXHAM 31st March EDINBURGH & THE LOTHIANS SHOW 14th April* PERTH SHOW 21st April HIGHLAND SHOW, NAIRN 28th April GLASGOW SHOW 5th May* ABERDEEN SHOW 19th May* GARDENING SCOTLAND (Joint Rock Only) 2nd June* LATE BULB DISPLAY, RBGE 8th September DISCUSSION WEEKEND, DUMFRIES 29th - 30th September NEWCASTLE SHOW 13th October* AGM 10th November† *Joint Rock Garden Plant Committee meetings 48 †Photographic/Art Competition SHOWS 2012 SHOW RULES 1. -

Temporal and Spatial Origin of Gesneriaceae in the New World Inferred from Plastid DNA Sequences
bs_bs_banner Botanical Journal of the Linnean Society, 2013, 171, 61–79. With 3 figures Temporal and spatial origin of Gesneriaceae in the New World inferred from plastid DNA sequences MATHIEU PERRET1*, ALAIN CHAUTEMS1, ANDRÉA ONOFRE DE ARAUJO2 and NICOLAS SALAMIN3,4 1Conservatoire et Jardin botaniques de la Ville de Genève, Ch. de l’Impératrice 1, CH-1292 Chambésy, Switzerland 2Centro de Ciências Naturais e Humanas, Universidade Federal do ABC, Rua Santa Adélia, 166, Bairro Bangu, Santo André, Brazil 3Department of Ecology and Evolution, University of Lausanne, CH-1015 Lausanne, Switzerland 4Swiss Institute of Bioinformatics, Quartier Sorge, CH-1015 Lausanne, Switzerland Received 15 December 2011; revised 3 July 2012; accepted for publication 18 August 2012 Gesneriaceae are represented in the New World (NW) by a major clade (c. 1000 species) currently recognized as subfamily Gesnerioideae. Radiation of this group occurred in all biomes of tropical America and was accompanied by extensive phenotypic and ecological diversification. Here we performed phylogenetic analyses using DNA sequences from three plastid loci to reconstruct the evolutionary history of Gesnerioideae and to investigate its relationship with other lineages of Gesneriaceae and Lamiales. Our molecular data confirm the inclusion of the South Pacific Coronanthereae and the Old World (OW) monotypic genus Titanotrichum in Gesnerioideae and the sister-group relationship of this subfamily to the rest of the OW Gesneriaceae. Calceolariaceae and the NW genera Peltanthera and Sanango appeared successively sister to Gesneriaceae, whereas Cubitanthus, which has been previously assigned to Gesneriaceae, is shown to be related to Linderniaceae. Based on molecular dating and biogeographical reconstruction analyses, we suggest that ancestors of Gesneriaceae originated in South America during the Late Cretaceous. -

Amazon Alive: a Decade of Discoveries 1999-2009
Amazon Alive! A decade of discovery 1999-2009 The Amazon is the planet’s largest rainforest and river basin. It supports countless thousands of species, as well as 30 million people. © Brent Stirton / Getty Images / WWF-UK © Brent Stirton / Getty Images The Amazon is the largest rainforest on Earth. It’s famed for its unrivalled biological diversity, with wildlife that includes jaguars, river dolphins, manatees, giant otters, capybaras, harpy eagles, anacondas and piranhas. The many unique habitats in this globally significant region conceal a wealth of hidden species, which scientists continue to discover at an incredible rate. Between 1999 and 2009, at least 1,200 new species of plants and vertebrates have been discovered in the Amazon biome (see page 6 for a map showing the extent of the region that this spans). The new species include 637 plants, 257 fish, 216 amphibians, 55 reptiles, 16 birds and 39 mammals. In addition, thousands of new invertebrate species have been uncovered. Owing to the sheer number of the latter, these are not covered in detail by this report. This report has tried to be comprehensive in its listing of new plants and vertebrates described from the Amazon biome in the last decade. But for the largest groups of life on Earth, such as invertebrates, such lists do not exist – so the number of new species presented here is no doubt an underestimate. Cover image: Ranitomeya benedicta, new poison frog species © Evan Twomey amazon alive! i a decade of discovery 1999-2009 1 Ahmed Djoghlaf, Executive Secretary, Foreword Convention on Biological Diversity The vital importance of the Amazon rainforest is very basic work on the natural history of the well known. -

Redalyc.Pollination Biology and Reproduction of Seemannia Sylvatica
Biota Neotropica ISSN: 1676-0611 [email protected] Instituto Virtual da Biodiversidade Brasil Camargo, Eduardo; da Cruz Rodrigues, Licléia; Cardoso Araujo, Andréa Pollination biology and reproduction of Seemannia sylvatica (Kunth) Hanstein (Gesneriaceae) in the Serra da Bodoquena National Park, Mato Grosso do Sul Biota Neotropica, vol. 11, núm. 4, 2011, pp. 125-130 Instituto Virtual da Biodiversidade Campinas, Brasil Available in: http://www.redalyc.org/articulo.oa?id=199122242013 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Biota Neotrop., vol. 11, no. 4 Pollination biology and reproduction of Seemannia sylvatica (Kunth) Hanstein (Gesneriaceae) in the Serra da Bodoquena National Park, Mato Grosso do Sul Eduardo Camargo1, Licléia da Cruz Rodrigues1,2 & Andréa Cardoso Araujo1,3 1Laboratório de Ecologia, Centro de Ciências Biológicas e da Saúde, Universidade Federal de Mato Grosso do Sul – UFMS, CP 549, CEP 79070-900, Campo Grande, MS, Brasil 2Laboratório de Ornitologia, Departamento de Zoologia, Instituto de Ciências Biológicas, Universidade Federal de Minas Gerais –UFMG, CP 486, CEP 31270-901, Belo Horizonte, MG, Brasil 3Corresponding author: Andréa Cardoso Araujo, e-mail: [email protected] CAMARGO, E., RODRIGUES, L.C. & ARAUJO, A.C. Pollination biology and reproduction of Seemannia sylvatica (Kunth) Hanstein (Gesneriaceae) in the Serra da Bodoquena National Park, Mato Grosso do Sul. Biota Neotrop. 11(4): http://www.biotaneotropica.org.br/v11n4/en/abstract?article+bn02911042011 Abstract: In Brazil, the family Gesneriaceae is represented by 23 genera and approximately 200 species. -

Anisocotyly and Meristem Initiation in an Unorthodox Plant, Streptocarpus Rexii (Gesneriaceae)
Planta (2007) 225:653–663 DOI 10.1007/s00425-006-0389-7 ORIGINAL ARTICLE Anisocotyly and meristem initiation in an unorthodox plant, Streptocarpus rexii (Gesneriaceae) RaVaella Mantegazza · Michael Möller · C. Jill Harrison · Simone Fior · Chiara De Luca · Alberto Spada Received: 26 April 2006 / Accepted: 22 August 2006 / Published online: 15 September 2006 © Springer-Verlag 2006 Abstract In common with most Old World Gesneria- ing post-embryonic development on the axis (the pet- ceae; Streptocarpus Lindl. shows anisocotylous growth, iolode) between the two cotyledons. The expression i.e., the continuous growth of one cotyledon after ger- pattern of SrSTM1 suggests a function in maintaining mination. Linked to this phenomenon is an unortho- cell division activity in the cotyledons before becoming dox behaviour of the shoot apical meristem (SAM) localized in the basal meristem, initially at the proximal that determines the growth pattern of acaulescent spe- ends of both cotyledons, later at the base of the contin- cies (subgenus Streptocarpus). In contrast caulescent uously growing macrocotyledon, and the groove meri- species develop a conventional central post-embryonic stem on the petiolode. The latter is equivalent to a SAM (mainly subgenus Streptocarpella). We used S. displaced SAM seemingly originating de novo under rexii Lindl. as a model to investigate anisocotyly and the inXuence of endogenous factors. Applied cytokinin meristem initiation in Streptocarpus by using histologi- retains SrSTM1expression in the small cotyledon, thus cal techniques and analyses of the expression pattern promoting isocotyly and re-establishment of a central of the meristematic marker SrSTM1 during ontogeny. post-embryonic SAM. Hormone-dependent delocal- In contrast to Arabidopsis thaliana (L.) Heynh., S. -

Palinotaxonomia De Espécies Brasileiras De Gesneriaceae, Com Ênfase Nas Ocorrentes No Estado De São Paulo
EDUARDO CUSTÓDIO GASPARINO Palinotaxonomia de espécies brasileiras de Gesneriaceae, com ênfase nas ocorrentes no Estado de São Paulo Tese apresentada ao Instituto de Botânica da Secretaria do Meio Ambiente, como parte dos requisitos exigidos para a obtenção do título de DOUTOR em BIODIVERSIDADE VEGETAL E MEIO AMBIENTE, na Área de Concentração de Plantas Vasculares em Análises Ambientais. SÃO PAULO 2008 EDUARDO CUSTÓDIO GASPARINO Palinotaxonomia de espécies brasileiras de Gesneriaceae, com ênfase nas ocorrentes no Estado de São Paulo Tese apresentada ao Instituto de Botânica da Secretaria do Meio Ambiente, como parte dos requisitos exigidos para a obtenção do título de DOUTOR em BIODIVERSIDADE VEGETAL E MEIO AMBIENTE, na Área de Concentração de Plantas Vasculares em Análises Ambientais. ORIENTADORA: DRA. MARIA AMÉLIA VITORINO DA CRUZ-BARROS CO-ORIENTADOR: DR. ALAIN CHAUTEMS Ficha Catalográfica elaborada pela Seção de Biblioteca do Instituto de Botânica Gasparino, Eduardo Custódio G249p Palinotaxonomia de espécies brasileiras de Gesneriaceae, com ênfase nas ocorrentes no Estado de São Paulo / Eduardo Custódio Gasparino -- São Paulo, 2008. 197 p.il. Tese (Doutorado) -- Instituto de Botânica da Secretaria de Estado do Meio Ambiente, 2008 Bibliografia. 1. Pólen. 2. Palinotaxonomia. 3. Gesneriaceae. I. Título CDU : 581.33 Alfa, Ômega... princípio e fim, sim Ele é... sim Ele é.... Lírio dos vales, estrela da manhã, para sempre cantarei o Seu amor!!! À Ele a glória, À Ele o louvor, à Ele o domínio... Ele é o Senhor Aos meus pais, Luzia Custódia Pereira Gasparino e Francisco Gasparino, dedico. À minha Orientadora Dra. Maria Amélia Obrigado por todos os ensinamentos, pela amizade, dedicação e pela orientação de todos estes anos e em especial nesta Tese. -

Rock Garden Quarterly
ROCK GARDEN QUARTERLY VOLUME 53 NUMBER 1 WINTER 1995 COVER: Aquilegia scopulorum with vespid wasp by Cindy Nelson-Nold of Lakewood, Colorado All Material Copyright © 1995 North American Rock Garden Society ROCK GARDEN QUARTERLY BULLETIN OF THE NORTH AMERICAN ROCK GARDEN SOCIETY formerly Bulletin of the American Rock Garden Society VOLUME 53 NUMBER 1 WINTER 1995 FEATURES Alpine Gesneriads of Europe, by Darrell Trout 3 Cassiopes and Phyllodoces, by Arthur Dome 17 Plants of Mt. Hutt, a New Zealand Preview, by Ethel Doyle 29 South Africa: Part II, by Panayoti Kelaidis 33 South African Sampler: A Dozen Gems for the Rock Garden, by Panayoti Kelaidis 54 The Vole Story, by Helen Sykes 59 DEPARTMENTS Plant Portrait 62 Books 65 Ramonda nathaliae 2 ROCK GARDEN QUARTERLY VOL. 53:1 ALPINE GESNERIADS OF EUROPE by Darrell Trout J. he Gesneriaceae, or gesneriad Institution and others brings the total family, is a diverse family of mostly Gesneriaceae of China to a count of 56 tropical and subtropical plants with genera and about 413 species. These distribution throughout the world, should provide new horticultural including the north and south temper• material for the rock garden and ate and tropical zones. The 125 genera, alpine house. Yet the choicest plants 2850-plus species include terrestrial for the rock garden or alpine house and epiphytic herbs, shrubs, vines remain the European genera Ramonda, and, rarely, small trees. Botanically, Jancaea, and Haberlea. and in appearance, it is not always easy to separate the family History Gesneriaceae from the closely related The family was named for Konrad Scrophulariaceae (Verbascum, Digitalis, von Gesner, a sixteenth century natu• Calceolaria), the Orobanchaceae, and ralist. -

Progress on Southeast Asia's Flora Projects
Gardens' Bulletin Singapore 71 (2): 267–319. 2019 267 doi: 10.26492/gbs71(2).2019-02 Progress on Southeast Asia’s Flora projects D.J. Middleton1, K. Armstrong2, Y. Baba3, H. Balslev4, K. Chayamarit5, R.C.K. Chung6, B.J. Conn7, E.S. Fernando8, K. Fujikawa9, R. Kiew6, H.T. Luu10, Mu Mu Aung11, M.F. Newman12, S. Tagane13, N. Tanaka14, D.C. Thomas1, T.B. Tran15, T.M.A. Utteridge16, P.C. van Welzen17, D. Widyatmoko18, T. Yahara14 & K.M. Wong1 1Singapore Botanic Gardens, National Parks Board, 1 Cluny Road, 259569 Singapore [email protected] 2New York Botanical Garden, 2900 Southern Boulevard, Bronx, New York, 10458, USA 3Auckland War Memorial Museum Tāmaki Paenga Hira, Private Bag 92018, Auckland 1142, New Zealand 4Ecoinformatics and Biodiversity, Department of Bioscience, Aarhus University Building 1540, Ny Munkegade 114, Aarhus C DK 8000, Denmark 5The Forest Herbarium, National Park, Wildlife and Plant Conservation Department, 61 Phahonyothin Rd., Chatuchak, Bangkok 10900, Thailand 6Herbarium, Forest Research Institute Malaysia, Kepong, Selangor 52109, Malaysia 7School of Life and Environmental Sciences, University of Sydney, NSW 2006, Australia 8Department of Forest Biological Sciences, College of Forestry & Natural Resources, University of the Philippines - Los Baños, College, 4031 Laguna, Philippines 9Kochi Prefectural Makino Botanical Garden, 4200-6 Godaisan, Kochi, 7818125, Japan 10Southern Institute of Ecology, Vietnam Academy of Science and Technology, 01 Mac Dinh Chi Street, District 1, Ho Chi Minh City, Vietnam 11Forest -

Gesneriads First Quarter 2018
GesThe Journal forn Gesneriade Growersria ds Volume 68 ~ Number 1 First Quarter 2018 Return to Table of Contents RETURN TO TABLE OF CONTENTS The Journal for Gesneriad Growers Volume 68 ~ Number 1 Gesneriads First Quarter 2018 FEATURES DEPARTMENTS 5 Saintpaulia, the NEW Streptocarpus 3 Message from the President Winston Goretsky Julie Mavity-Hudson 9 Style Guide for Writers 4 From The Editor Jeanne Katzenstein Peter Shalit 10 Gesneriads at the Liuzhou Arts Center 18 Gesneriad Registrations Wallace Wells Irina Nicholson 24 Flower Show Awards 42 Changes to Hybrid Seed List 4Q17 Paul Susi Gussie Farrice 25 Gesneriads POP in New England! 46 Coming Events Maureen Pratt Ray Coyle and Karyn Cichocki 28 62nd Annual Convention of The 47 Flower Show Roundup Gesneriad Society 51 Back to Basics: Gesneriad Crafts 37 Convention Speakers Dale Martens Dee Stewart 55 Seed Fund – Species 39 Petrocosmeas in the United Kingdom Carolyn Ripps Razvan Chisu 61 Information about The Gesneriad 43 Gasteranthus herbaceus – A white- Society, Inc. flowered Gasteranthus from the northern Andes Dale Martens with John L. Clark Cover Eucodonia ‘Adele’ grown by Eileen McGrath Back Cover and exhibited at the New York State African Petrocosmea ‘Stone Amethyst’, hybridized, Violet Convention Show, October 2017. grown, and photographed by Andy Kuang. Photo: Bob Clark See New Registrations article, page 18. Editor Business Manager The Gesneriad Society, Inc. Peter Shalit Michael A. Riley The objects of The Gesneriad [email protected] [email protected] Society are to afford