Chemistry 101 2-MEASUREMENT: VOLUME, MASS, and DENSITY
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Determination of the Identity of an Unknown Liquid Group # My Name the Date My Period Partner #1 Name Partner #2 Name
Determination of the Identity of an unknown liquid Group # My Name The date My period Partner #1 name Partner #2 name Purpose: The purpose of this lab is to determine the identity of an unknown liquid by measuring its density, melting point, boiling point, and solubility in both water and alcohol, and then comparing the results to the values for known substances. Procedure: 1) Density determination Obtain a 10mL sample of the unknown liquid using a graduated cylinder Determine the mass of the 10mL sample Save the sample for further use 2) Melting point determination Set up an ice bath using a 600mL beaker Obtain a ~5mL sample of the unknown liquid in a clean dry test tube Place a thermometer in the test tube with the sample Place the test tube in the ice water bath Watch for signs of crystallization, noting the temperature of the sample when it occurs Save the sample for further use 3) Boiling point determination Set up a hot water bath using a 250mL beaker Begin heating the water in the beaker Obtain a ~10mL sample of the unknown in a clean, dry test tube Add a boiling stone to the test tube with the unknown Open the computer interface software, using a graph and digit display Place the temperature sensor in the test tube so it is in the unknown liquid Record the temperature of the sample in the test tube using the computer interface Watch for signs of boiling, noting the temperature of the unknown Dispose of the sample in the assigned waste container 4) Solubility determination Obtain two small (~1mL) samples of the unknown in two small test tubes Add an equal amount of deionized into one of the samples Add an equal amount of ethanol into the other Mix both samples thoroughly Compare the samples for solubility Dispose of the samples in the assigned waste container Observations: The unknown is a clear, colorless liquid. -

Laboratory Equipment Reference Sheet
Laboratory Equipment Stirring Rod: Reference Sheet: Iron Ring: Description: Glass rod. Uses: To stir combinations; To use in pouring liquids. Evaporating Dish: Description: Iron ring with a screw fastener; Several Sizes Uses: To fasten to the ring stand as a support for an apparatus Description: Porcelain dish. Buret Clamp/Test Tube Clamp: Uses: As a container for small amounts of liquids being evaporated. Glass Plate: Description: Metal clamp with a screw fastener, swivel and lock nut, adjusting screw, and a curved clamp. Uses: To hold an apparatus; May be fastened to a ring stand. Mortar and Pestle: Description: Thick glass. Uses: Many uses; Should not be heated Description: Heavy porcelain dish with a grinder. Watch Glass: Uses: To grind chemicals to a powder. Spatula: Description: Curved glass. Uses: May be used as a beaker cover; May be used in evaporating very small amounts of Description: Made of metal or porcelain. liquid. Uses: To transfer solid chemicals in weighing. Funnel: Triangular File: Description: Metal file with three cutting edges. Uses: To scratch glass or file. Rubber Connector: Description: Glass or plastic. Uses: To hold filter paper; May be used in pouring Description: Short length of tubing. Medicine Dropper: Uses: To connect parts of an apparatus. Pinch Clamp: Description: Glass tip with a rubber bulb. Uses: To transfer small amounts of liquid. Forceps: Description: Metal clamp with finger grips. Uses: To clamp a rubber connector. Test Tube Rack: Description: Metal Uses: To pick up or hold small objects. Beaker: Description: Rack; May be wood, metal, or plastic. Uses: To hold test tubes in an upright position. -

LOWERING COSTS and SIMPLIFYING WORKFLOW with the Automate 2550 SYSTEM
HACETTEPE UNIVERSITY FACULTY OF MEDICINE HOSPITAL CENTRAL AND STAT LABORATORIES LOWERING COSTS AND SIMPLIFYING WORKFLOW WITH THE AutoMate 2550 SYSTEM Laboratory profile › 1,040-bed hospital located in Ankara, Turkey › Provides uninterrupted 24/7 inpatient, outpatient and emergency services › Employs 6 professors, 6 associate Hacettepe University Faculty of Medicine Hospital is one of the leading professors and 130 operators university hospitals in Turkey. The university is well known for its › Performs up to 6.5 million geographical location and adoption of ground breaking technologies. biochemistry and hormone While the reference site is preferred by a large number of patients, it is tests annually of particular interest to specific patient populations seeking test results › Processes up to 2 million tests from a variety of panels. With many different systems performing on instruments in more than hundreds of standard and specialized tests, the hospital’s laboratories 20 different units are driven to ensure the delivery of results without error, while › Operates a Beckman Coulter containing costs and managing workload burden. standalone AutoMate 2550 pre- For more than 16 years, the facility—which has been held up as an and post-analytical automation system in the biochemistry example of excellence in its own region, as well as all over the country— laboratory, along with a Power has been using a standalone automation system with an aliquoter Processor system with two feature. With this technology, it has enhanced patient satisfaction, -
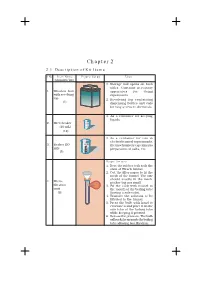
Chapter 2 2.1 Description of Kit Items
Manual of SEAT Friendly MCL Kit 7 Chapter 2 2.1 Description of Kit Items S.No. Item Name Figure/Setup Uses (Quantity/ kit) 1. Storage box opens on both sides. Contains necessary 1. Wooden box apparatus for doing with revolving experiments. top 2. Revolving top containing (1) dispensing bottles and vials for easy access to chemicals. 1. As a container for keeping liquids. 2. Microbeaker (10 mL) (12) 1. As a container for use in electrochemical experiments, 3. Beaker (50 thermochemistry experiments mL) preparation of salts, etc. (3) Steps for use 1. Bore the rubber cork to fit the stem of Hirsch funnel. 2. Cut the filter paper to fit the mesh of the funnel. The size should exactly fit the mesh, 4. Micro- neither big nor small. filtration 3. Fit the cork with funnel in unit the mouth of the boiling tube (2) (having a side tube). 4. Transfer the solution to be filtrated to the funnel. 5. Press the bulb with hand to evacuate it and place it on the side tube of the boiling tube while keeping it pressed. 6. Release the pressure. The bulb will suck the air inside the boiling tube allowing fast filtration. 8 Manual of SEAT Friendly MCL Kit S.No. Item Name Figure/Setup Uses (Quantity/Kit) 1. The watch glass is used to hold solids when being 5. Watch glass weighed. (2) 2. It should never be heated. 1. Flat plate with multiple “wells” used as micro test tubes. 2. Contains 96 rectangular matrix. 3. Each well of a micro plate typically holds somewhere between a few to a few hundred microlitres of liquid. -

Laboratory Supplies and Equipment
Laboratory Supplies and Equipment Beakers: 9 - 12 • Beakers with Handles • Printed Square Ratio Beakers • Griffin Style Molded Beakers • Tapered PP, PMP & PTFE Beakers • Heatable PTFE Beakers Bottles: 17 - 32 • Plastic Laboratory Bottles • Rectangular & Square Bottles Heatable PTFE Beakers Page 12 • Tamper Evident Plastic Bottles • Concertina Collapsible Bottle • Plastic Dispensing Bottles NEW Straight-Side Containers • Plastic Wash Bottles PETE with White PP Closures • PTFE Bottle Pourers Page 39 Containers: 38 - 42 • Screw Cap Plastic Jars & Containers • Snap Cap Plastic Jars & Containers • Hinged Lid Plastic Containers • Dispensing Plastic Containers • Graduated Plastic Containers • Disposable Plastic Containers Cylinders: 45 - 48 • Clear Plastic Cylinder, PMP • Translucent Plastic Cylinder, PP • Short Form Plastic Cylinder, PP • Four Liter Plastic Cylinder, PP NEW Polycarbonate Graduated Bottles with PP Closures Page 21 • Certified Plastic Cylinder, PMP • Hydrometer Jar, PP • Conical Shape Plastic Cylinder, PP Disposal Boxes: 54 - 55 • Bio-bin Waste Disposal Containers • Glass Disposal Boxes • Burn-upTM Bins • Plastic Recycling Boxes • Non-Hazardous Disposal Boxes Printed Cylinders Page 47 Drying Racks: 55 - 56 • Kartell Plastic Drying Rack, High Impact PS • Dynalon Mega-Peg Plastic Drying Rack • Azlon Epoxy Coated Drying Rack • Plastic Draining Baskets • Custom Size Drying Racks Available Burn-upTM Bins Page 54 Dynalon® Labware Table of Contents and Introduction ® Dynalon Labware, a leading wholesaler of plastic lab supplies throughout -

High School Chemistry
RECOMMENDED MINIMUM CORE INVENTORY TO SUPPORT STANDARDS-BASED INSTRUCTION HIGH SCHOOL GRADES SCIENCES High School Chemistry Quantity per Quantity per lab classroom/ Description group adjacent work area SAFETY EQUIPMENT 2 Acid storage cabinet (one reserved exclusively for nitric acid) 1 Chemical spill kit 1 Chemical storage reference book 5 Chemical waste containers (Categories: corrosives, flammables, oxidizers, air/water reactive, toxic) 1 Emergency shower 1 Eye wash station 1 Fire blanket 1 Fire extinguisher 1 First aid kit 1 Flammables cabinet 1 Fume hood 1/student Goggles 1 Goggles sanitizer (holds 36 pairs of goggles) 1/student Lab aprons COMPUTER ASSISTED LEARNING 1 Television or digital projector 1 VGA Adapters for various digital devices EQUIPMENT/SUPPLIES 1 box Aluminum foil 100 Assorted rubber stoppers 1 Balance, analytical (0.001g precision) 5 Balance, electronic or manual (0.01g precision) 1 pkg of 50 Balloons, latex 4 Beakers, 50 mL 4 Beakers, 100 mL 2 Beakers, 250 mL Developed by California Science Teachers Association to support the implementation of the California Next Generation Science Standards. Approved by the CSTA Board of Directors November 17, 2015. Quantity per Quantity per lab classroom/ Description group adjacent work area 2 Beakers, 400 or 600 mL 1 Beakers, 1000 mL 1 Beaker tongs 1 Bell jar 4 Bottle, carboy round, LDPE 10 L 4 Bottle, carboy round, LDPE 4 L 10 Bottle, narrow mouth, 1000 mL 20 Bottle, narrow mouth, 125 mL 20 Bottle, narrow mouth, 250 mL 20 Bottle, narrow mouth, 500 mL 10 Bottle, wide mouth, 125 -

When Magnesium Metal Reacts with Hydrochloric Acid, Hydrogen Gas Is Produced
Clements—Chemistry I H Molar Volume of a Gas When magnesium metal reacts with hydrochloric acid, hydrogen gas is produced. The volume of this gas can be measured by using a eudiometer. A eudiometer is a giant test tube that has graduated volume measurements. Knowing the number of moles of magnesium used, you can calculate the volume of hydrogen produced per mole of magnesium consumed. The balanced equation for this reaction also allows you to determine the volume that one mole of gas occupies as a recorded temperature and pressure. Procedure Half fill a tall cylinder with tap water, and place about 1O0mL of tap water in a 25O-mL beaker. Obtain ~1OmL of 3M hydrochloric acid in a graduated cylinder. Acquire a piece of magnesium ribbon and measure and record its mass to the nearest 0.OOlg. Roll the magnesium ribbon into a loose coil. Tie the coil with a piece of thread in such a manner that all of the loops of the coil are tied together. With a funnel, carefully pour ~10mL of 3M HCl (aq) into the eudiometer. Using the room temperature water in the 250-mL beaker, very slowly pour water from the beaker into the eudiometer which is in a slightly tipped position. Be careful to layer the water over the acid so that they do not mix thoroughly. Add enough water to fill the eudiometer completely. Using the thread, lower the magnesium coil into the water in the eudiometer to a depth of ~5cm. Insert the rubber stopper into the mouth of the eudiometer to hold the thread in position. -

Microbiology. Applied to Protecting the Line
3M Food Safety Division Microbiology. Applied to protecting the line www.3M.com/foodsafety/us Increase Productivity & Profitability to Your Business by 3M Food Safety Product Cost 45% Product Cost Saving Increase Productivity • 45% Reducing labor cost, saving utilities • Ability to increase productivity in food processing. and equipments of sample preparation 3M rapid solution help to detect contamination, • The shelf-stable, thin design takes up 85% less space faster time to results. Making faster decision. than agar dishes, freeing incubator and storage space and significantly reducing biohazardous waste • Control the production process better than ever. Higher product quality and Reduce overall costs, such as reducing waste, reducing Break down. OFF ON Fas te ISO r HACCP GMP Brand Protection Inventory Management • Establish brand credibility. Reliable • Release product to the hands of consumers faster. testing results with the global standard Company faster gaining profit • Reduce risk of product from the Recall • Reduce cost of storage inventory concerns. and Reject. Making trust from business partners. 2 Increase Laboratory Productivity by 3M Food Safety Product Product 3M Food Safety Cost 45% Cost Saving Accuracy • 45% Reduce the cost of labor, • Provide accurate results to Make decisions saving utilities and equipments correctly. Increase effectiveness of laboratory for sample preparation. • Build the credibility of laboratory with your • Reduce the cost of acquisition lab partners and auditors with the international equibments or the expansion of the method standards laboratory. Space less than 85% of Agar. Time 50% Software Time • Faster time to result.The product is designed to reduce steps and timing compared to the traditional method. -

3M™ Petrifilm™ Aerobic Count Plates Introduction SCIENTIFIC Petrifilm™ Plates Are Designed to Determine Total Aerobic Bacterial Populations
3M™ Petrifilm™ Aerobic Count Plates Introduction SCIENTIFIC Petrifilm™ plates are designed to determine total aerobic bacterial populations. The easy-to-use BIO FAX! Petrifilm plates contain a film coated with nutrients and gelling agents. You will be able to monitor environmental bacterial counts using a variety of methods. Three environmental sampling methods are described as well as the standard inoculation procedure. Concepts • Microbiology • Plate counting Materials Petrifilm Aerobic Count Plates Incubator Petrifilm Spreader Pipet Chip clips Tape, double-sided Cotton swab, sterile Test tube, screwtop Safety Precautions Wear chemical-resistant gloves while working with microbial organisms. After use, Petrifilm Aerobic Count Plates will contain viable cultures. Do NOT reopen the Petrifilm plates or handle unnecessarily. Wash hands thoroughly with soap and water before leaving the laboratory. Extreme caution should be exercised when handling culture materials, and students should be trained in aseptic techniques. Always clean work areas and wash hands after working with microbiological materials. Procedures Standard Inoculation 1. Place the Petrifilm Aerobic Count Plate on a flat surface. Carefully peel open the Petrifilm plate being careful not to touch the nutrient gel with your fingers. (See Figure 1.) 2. With a pipet perpendicular to the Petrifilm plate, place 1 mL of sample (inoculum) onto the center of the bottom film. (See Figure 2.) 3. Release the top film; allow it to drop. Do not roll top film down. (See Figure 3.) Figure 1. Figure 2. Figure 3. FLAT side RIDGE side Figure 4. Figure 5. Figure 6. © 2016 Flinn Scientific, Inc. All Rights Reserved. Publication No. 10163 1 061616 3M™ Petrifilm™ Aerobic Count Plates continued 4. -

STERILE TECHNIQUE PRACTICE Module 3 Supplement
STUDENT STERILE TECHNIQUE PRACTICE Module 3 Supplement ONLY FOR PRACTICE you should Re-use the loop and pipette and re-use their "sterile" wrappings Do not dispose of a culture dish even if you accidentally contaminate it. If you were really doing a lab with bacteria you would • Never reuse any loop or pipette. • Always dispose of used loops and pipettes in the "biological trash beaker." • Throw away any culture dish or other item that is accidentally contaminated, and ask for a replacement. • Wash hands immediately if they contact any liquids containing bacteria. Each member of the team should practice each of the following: 1. Open, hold, and close a sterile 15 ml culture tube. 2. Open and close a sterile 1.5 ml test tube. 3. Open a culture dish, observe it without breathing on it, and close it. After each team member has practiced 1-3, use (practice) sterile technique as you 4. Transfer 250 l of liquid from the 1.5 ml test tube to the culture tube. 5. Pretend to transfer a colony from the surface of the culture dish to the liquid in the culture tube. 6. Use the pipette to suspend the imaginary bacteria in the culture tube liquid. After each team member has practiced 4-6, use (practice) sterile technique as you 7. Transfer 100 l of culture tube contents to culture dish and spread it. POSSIBLE SEQUENCE OF STEPS FOR STERILE TECHNIQUE DEMONSTRATION/PRACTICE Delete parts as time requires 1. a. Teacher demonstrates opening/holding/closing of sterile 1.5 ml test tube, sterile 15 ml culture tube, sterile pipettes, measuring with pipettes. -
Revolutionary Blood Test Tube 2.0
Revolutionary Blood Test Tube 2.0 Elmasry, Julian The newly designed test tube presented in this abstract would improve on the design of blood test tubes commonly used by hospitals, clinics, and both research and commercial laboratories all over the world. The design allowed drawn blood samples to stand warm climate over an extended period of time exceeding current commonly used blood-collecting test tubes. Since warm temperature alters and affects accuracy of blood test results, the currently used test tubes require refrigeration until blood is tested. The importance of current design would save the cost of refrigeration and transportation of refrigerated blood samples. The test tube is made of 3 sealed test tubes encasing each other with a gradual increase in diameter with the outermost tube having the biggest diameter. The space between the inner tube and intermediate tube contains Ammonium Thiocyanate and water to generate an endothermic reaction. The space between the intermediate and the third/outermost tube would contain a thin layer of insulation. The temperature of the blood sample of the experimental blood test tube was tested using electronic thermometer and an infrared gun. The results showed that the proposed experimental test tube, when compared to currently used tubes, successfully lowered the temperature of collected blood sample almost 9 to 11 degrees Celsius for about 4 to 5 hours longer. This design would save the cost of refrigeration and would allow delivery of adequate health care at a lower cost especially in locations with warm climates and 3rd world countries. Awards Won: Fourth Award of $500. -

Laboratory Equipment Used in Filtration
KNOW YOUR LAB EQUIPMENTS Test tube A test tube, also known as a sample tube, is a common piece of laboratory glassware consisting of a finger-like length of glass or clear plastic tubing, open at the top and closed at the bottom. Beakers Beakers are used as containers. They are available in a variety of sizes. Although they often possess volume markings, these are only rough estimates of the liquid volume. The markings are not necessarily accurate. Erlenmeyer flask Erlenmeyer flasks are often used as reaction vessels, particularly in titrations. As with beakers, the volume markings should not be considered accurate. Volumetric flask Volumetric flasks are used to measure and store solutions with a high degree of accuracy. These flasks generally possess a marking near the top that indicates the level at which the volume of the liquid is equal to the volume written on the outside of the flask. These devices are often used when solutions containing dissolved solids of known concentration are needed. Graduated cylinder Graduated cylinders are used to transfer liquids with a moderate degree of accuracy. Pipette Pipettes are used for transferring liquids with a fixed volume and quantity of liquid must be known to a high degree of accuracy. Graduated pipette These Pipettes are calibrated in the factory to release the desired quantity of liquid. Disposable pipette Disposable transfer. These Pipettes are made of plastic and are useful for transferring liquids dropwise. Burette Burettes are devices used typically in analytical, quantitative chemistry applications for measuring liquid solution. Differing from a pipette since the sample quantity delivered is changeable, graduated Burettes are used heavily in titration experiments.