Salt Stress Vs. Salt Shock
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

METABOLIC EVOLUTION in GALDIERIA SULPHURARIA By
METABOLIC EVOLUTION IN GALDIERIA SULPHURARIA By CHAD M. TERNES Bachelor of Science in Botany Oklahoma State University Stillwater, Oklahoma 2009 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY May, 2015 METABOLIC EVOLUTION IN GALDIERIA SUPHURARIA Dissertation Approved: Dr. Gerald Schoenknecht Dissertation Adviser Dr. David Meinke Dr. Andrew Doust Dr. Patricia Canaan ii Name: CHAD M. TERNES Date of Degree: MAY, 2015 Title of Study: METABOLIC EVOLUTION IN GALDIERIA SULPHURARIA Major Field: PLANT SCIENCE Abstract: The thermoacidophilic, unicellular, red alga Galdieria sulphuraria possesses characteristics, including salt and heavy metal tolerance, unsurpassed by any other alga. Like most plastid bearing eukaryotes, G. sulphuraria can grow photoautotrophically. Additionally, it can also grow solely as a heterotroph, which results in the cessation of photosynthetic pigment biosynthesis. The ability to grow heterotrophically is likely correlated with G. sulphuraria ’s broad capacity for carbon metabolism, which rivals that of fungi. Annotation of the metabolic pathways encoded by the genome of G. sulphuraria revealed several pathways that are uncharacteristic for plants and algae, even red algae. Phylogenetic analyses of the enzymes underlying the metabolic pathways suggest multiple instances of horizontal gene transfer, in addition to endosymbiotic gene transfer and conservation through ancestry. Although some metabolic pathways as a whole appear to be retained through ancestry, genes encoding individual enzymes within a pathway were substituted by genes that were acquired horizontally from other domains of life. Thus, metabolic pathways in G. sulphuraria appear to be composed of a ‘metabolic patchwork’, underscored by a mosaic of genes resulting from multiple evolutionary processes. -

Effects of Environmental Contaminants on Gene Expression, DNA Methylation and Gut Microbiota in Buff-Tailed Bumble Bee - Bombus Terrestris
UNIVERSITYOF LEICESTER DOCTORAL THESIS Effects of environmental contaminants on gene expression, DNA methylation and gut microbiota in Buff-tailed Bumble bee - Bombus terrestris Author: Supervisor: Pshtiwan BEBANE Eamonn MALLON A thesis submitted in fulfilment of the requirements for the degree of Doctor of Philosophy in the Department of Genetics and Genome Biology March 2019 Abstract Bee populations are increasingly at risk. In this thesis, I explore various mechanisms through which environmental contaminants, namely imidacloprid and black carbon, can affect bumble bee epigenetics, behaviour and gut microbiota. I found imidacloprid has numerous epigenetic effects on Bombus terrestris non reproductive workers. I analysed three whole methylome (BS-seq) libraries and seven RNA-seq libraries of the brains of imidacloprid exposed workers and three BS-seq libraries and nine RNA-seq li- braries from unexposed, control workers. I found 79, 86 and 16 genes differentially methylated at CpGs, CHHs and CHGs sites respectively between groups. I found CpG methylation much more focused in exon regions compared with methylation at CHH or CHG sites. I found 378 genes that were differentially expressed between imidacloprid treated and control bees. In ad- dition, I found 25 genes differentially alternatively spliced between control and imidacloprid samples. I used Drosophila melanogaster as a model for the behavioural effects of imidacloprid on in- sects. Imidacloprid did not affect flies’ periodicity. Low doses (2.5 ppb) of imidacloprid in- creased flies’ activity while high doses (20 ppb) decreased activity. Canton-S strain was more sensitive to imidacloprid during geotaxis assay than M1217. I proposed that a possible modulator of imidacloprid’s effects on insects is its effects on insects’ gut microbiota. -

Discovery of Novel Bacterial Queuine Salvage Enzymes and Pathways in Human Pathogens
Discovery of novel bacterial queuine salvage enzymes and pathways in human pathogens a,1 b,1 c,1 b d a Yifeng Yuan , Rémi Zallot , Tyler L. Grove , Daniel J. Payan , Isabelle Martin-Verstraete , Sara Sepic´ , Seetharamsingh Balamkundue, Ramesh Neelakandane, Vinod K. Gadie, Chuan-Fa Liue, Manal A. Swairjof,g, Peter C. Dedone,h,i, Steven C. Almoc, John A. Gerltb,j,k, and Valérie de Crécy-Lagarda,l,2 aDepartment of Microbiology and Cell Science, University of Florida, Gainesville, FL 32611; bInstitute for Genomic Biology, University of Illinois at Urbana–Champaign, Urbana, IL 61801; cDepartment of Biochemistry, Albert Einstein College of Medicine, Bronx, NY 10461; dLaboratoire de Pathogénèse des Bactéries Anaérobies, Institut Pasteur et Université de Paris, F-75015 Paris, France; eSingapore-MIT Alliance for Research and Technology, Infectious Disease Interdisciplinary Research Group, 138602 Singapore, Singapore; fDepartment of Chemistry and Biochemistry, San Diego State University, San Diego, CA 92182; gThe Viral Information Institute, San Diego State University, San Diego, CA 92182; hDepartment of Biological Engineering and Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139; iCenter for Environmental Health Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139; jDepartment of Biochemistry, University of Illinois at Urbana–Champaign, Urbana, IL 61801; kDepartment of Chemistry, University of Illinois at Urbana–Champaign, Urbana, IL 61801; and lUniversity of Florida Genetics Institute, Gainesville, FL 32610 Edited by Tina M. Henkin, The Ohio State University, Columbus, OH, and approved August 1, 2019 (received for review June 16, 2019) Queuosine (Q) is a complex tRNA modification widespread in 1A. The TGT enzyme, which is responsible for the base ex- eukaryotes and bacteria that contributes to the efficiency and accuracy change, is the signature enzyme in the Q biosynthesis pathway. -

Downloaded As a Text File, Is Completely Dynamic
BMC Bioinformatics BioMed Central Database Open Access ORENZA: a web resource for studying ORphan ENZyme activities Olivier Lespinet and Bernard Labedan* Address: Institut de Génétique et Microbiologie, CNRS UMR 8621, Université Paris-Sud, Bâtiment 400, 91405 Orsay Cedex, France Email: Olivier Lespinet - [email protected]; Bernard Labedan* - [email protected] * Corresponding author Published: 06 October 2006 Received: 25 July 2006 Accepted: 06 October 2006 BMC Bioinformatics 2006, 7:436 doi:10.1186/1471-2105-7-436 This article is available from: http://www.biomedcentral.com/1471-2105/7/436 © 2006 Lespinet and Labedan; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Despite the current availability of several hundreds of thousands of amino acid sequences, more than 36% of the enzyme activities (EC numbers) defined by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB) are not associated with any amino acid sequence in major public databases. This wide gap separating knowledge of biochemical function and sequence information is found for nearly all classes of enzymes. Thus, there is an urgent need to explore these sequence-less EC numbers, in order to progressively close this gap. Description: We designed ORENZA, a PostgreSQL database of ORphan ENZyme Activities, to collate information about the EC numbers defined by the NC-IUBMB with specific emphasis on orphan enzyme activities. -

(12) United States Patent (10) Patent No.: US 9,689,046 B2 Mayall Et Al
USOO9689046B2 (12) United States Patent (10) Patent No.: US 9,689,046 B2 Mayall et al. (45) Date of Patent: Jun. 27, 2017 (54) SYSTEM AND METHODS FOR THE FOREIGN PATENT DOCUMENTS DETECTION OF MULTIPLE CHEMICAL WO O125472 A1 4/2001 COMPOUNDS WO O169245 A2 9, 2001 (71) Applicants: Robert Matthew Mayall, Calgary (CA); Emily Candice Hicks, Calgary OTHER PUBLICATIONS (CA); Margaret Mary-Flora Bebeselea, A. et al., “Electrochemical Degradation and Determina Renaud-Young, Calgary (CA); David tion of 4-Nitrophenol Using Multiple Pulsed Amperometry at Christopher Lloyd, Calgary (CA); Lisa Graphite Based Electrodes', Chem. Bull. “Politehnica” Univ. Kara Oberding, Calgary (CA); Iain (Timisoara), vol. 53(67), 1-2, 2008. Fraser Scotney George, Calgary (CA) Ben-Yoav. H. et al., “A whole cell electrochemical biosensor for water genotoxicity bio-detection”. Electrochimica Acta, 2009, 54(25), 6113-6118. (72) Inventors: Robert Matthew Mayall, Calgary Biran, I. et al., “On-line monitoring of gene expression'. Microbi (CA); Emily Candice Hicks, Calgary ology (Reading, England), 1999, 145 (Pt 8), 2129-2133. (CA); Margaret Mary-Flora Da Silva, P.S. et al., “Electrochemical Behavior of Hydroquinone Renaud-Young, Calgary (CA); David and Catechol at a Silsesquioxane-Modified Carbon Paste Elec trode'. J. Braz. Chem. Soc., vol. 24, No. 4, 695-699, 2013. Christopher Lloyd, Calgary (CA); Lisa Enache, T. A. & Oliveira-Brett, A. M., "Phenol and Para-Substituted Kara Oberding, Calgary (CA); Iain Phenols Electrochemical Oxidation Pathways”, Journal of Fraser Scotney George, Calgary (CA) Electroanalytical Chemistry, 2011, 1-35. Etesami, M. et al., “Electrooxidation of hydroquinone on simply prepared Au-Pt bimetallic nanoparticles'. Science China, Chem (73) Assignee: FREDSENSE TECHNOLOGIES istry, vol. -

Functional Annotation of Uncharacterized Enzymes in Yeast
Functional Annotation of Uncharacterized Enzymes in Saccharomyces cerevisiae by Julia Ann Hanchard A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy Department of Molecular Genetics University of Toronto © Copyright by Julia Hanchard 2019 Abstract Functional Annotation of Uncharacterized Enzymes in Saccharomyces cerevisiae Julia Hanchard Doctor of Philosophy, 2019 Department of Molecular Genetics University of Toronto In the post-genomic era, clinicians and scientists are increasingly reliant on interpretation of variants in metabolic genes for determining pathogenicity. These interpretations depend on functional annotation of the roles genes provide in metabolism, an annotation that is far from complete. I embarked on a journey of enzyme discovery to fill gaps in our knowledge of metabolism in the budding yeast, Saccharomyces cerevisiae. I carried out a genetic and metabolomic screen of 120 uncharacterized candidate enzyme encoding genes that comprised my master’s thesis. This dissertation describes my work in ascribing function to two distinct enzymes, Das2 and Tda5. Throughout my study I have found that Das2 is a novel uridine/cytidine kinase that functions in concert with a second minor uridine kinase, Urk1. These two enzymes are interdependent and in turn depend on a third enzyme, the major uracil phosphoribosyl transferase, Fur1 for stability. These three enzymes form a complex that is essential to wild-type pyrimidine salvage. As I aimed to elucidate the function of Tda5, I discovered that this uncharacterized enzyme is essential to growth. Loss of function mutations in TDA5 are alleviated by de-repression of its sporulation specific paralog, Ydl114w, and when YDL114W is deleted, tda5Δ is rescued by hypomorphic mutations in the ergosterol biosynthetic pathway. -

Coupled Nucleoside Phosphorylase Reactions in Escherichia Coli John Lewis Ott Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1956 Coupled nucleoside phosphorylase reactions in Escherichia coli John Lewis Ott Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Biochemistry Commons, and the Microbiology Commons Recommended Citation Ott, John Lewis, "Coupled nucleoside phosphorylase reactions in Escherichia coli " (1956). Retrospective Theses and Dissertations. 13758. https://lib.dr.iastate.edu/rtd/13758 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NOTE TO USERS This reproduction is the best copy available. UMI COUPLED NUCLEOSIDE PHOSPHORYLASE REACTIONS IN ESCHERICHIA COLI / by John Lewis Ott A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subject: Physlolgglcal Bacteriology Approved: Signature was redacted for privacy. In Charge of Major Work Signature was redacted for privacy. Head of Major Department Signature was redacted for privacy. Dean of Graduate College Iowa State College 1956 UMI Number: DP12892 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. -

(12) United States Patent (10) Patent No.: US 8,962,800 B2 Mathur Et Al
USOO89628OOB2 (12) United States Patent (10) Patent No.: US 8,962,800 B2 Mathur et al. (45) Date of Patent: Feb. 24, 2015 (54) NUCLEICACIDS AND PROTEINS AND USPC .......................................................... 530/350 METHODS FOR MAKING AND USING THEMI (58) Field of Classification Search None (75) Inventors: Eric J. Mathur, San Diego, CA (US); See application file for complete search history. Cathy Chang, San Diego, CA (US) (56) References Cited (73) Assignee: BP Corporation North America Inc., Naperville, IL (US) PUBLICATIONS (*) Notice: Subject to any disclaimer, the term of this Nolling etal (J. Bacteriol. 183: 4823 (2001).* patent is extended or adjusted under 35 Spencer et al., “Whole-Genome Sequence Variation among Multiple U.S.C. 154(b) by 0 days. Isolates of Pseudomonas aeruginosa J. Bacteriol. (2003) 185: 1316-1325. (21) Appl. No.: 13/400,365 2002.Database Sequence GenBank Accession No. BZ569932 Dec. 17. 1-1. Mount, Bioinformatics, Cold Spring Harbor Press, Cold Spring Har (22) Filed: Feb. 20, 2012 bor New York, 2001, pp. 382-393. O O Omiecinski et al., “Epoxide Hydrolase-Polymorphism and role in (65) Prior Publication Data toxicology” Toxicol. Lett. (2000) 1.12: 365-370. US 2012/O266329 A1 Oct. 18, 2012 * cited by examiner Related U.S. Application Data - - - Primary Examiner — James Martinell (62) Division of application No. 1 1/817,403, filed as (74) Attorney, Agent, or Firm — DLA Piper LLP (US) application No. PCT/US2006/007642 on Mar. 3, 2006, now Pat. No. 8,119,385. (57) ABSTRACT (60) Provisional application No. 60/658,984, filed on Mar. The invention provides polypeptides, including enzymes, 4, 2005. -
![[Frontiers in Bioscience 7, D1762-1781, August 1, 2002] 1762 the NECESSITY of COMBINING GENOMIC and ENZYMATIC DATA to INFER META](https://docslib.b-cdn.net/cover/7446/frontiers-in-bioscience-7-d1762-1781-august-1-2002-1762-the-necessity-of-combining-genomic-and-enzymatic-data-to-infer-meta-1917446.webp)
[Frontiers in Bioscience 7, D1762-1781, August 1, 2002] 1762 the NECESSITY of COMBINING GENOMIC and ENZYMATIC DATA to INFER META
[Frontiers in Bioscience 7, d1762-1781, August 1, 2002] THE NECESSITY OF COMBINING GENOMIC AND ENZYMATIC DATA TO INFER METABOLIC FUNCTION AND PATHWAYS IN THE SMALLEST BACTERIA: AMINO ACID, PURINE AND PYRIMIDINE METABOLISM IN MOLLICUTES J. Dennis Pollack Department of Molecular Virology, Immunology and Medical Genetics, The College of Medicine and Public Health, The Ohio State University, 333 West 10th Avenue, Columbus, OH 43210 TABLE OF CONTENTS 1. Abstract 2. Introduction 3. Metabolic Linkages to Amino Acids and Proteins 4. Amino Acids: Transport 5. Amino Acids: Biosynthesis and Metabolism 6. Amino Acids: Aromatic amino acid synthesis in Acholeplasma-Anaeroplasma 7. Purine Metabolism: Transport 8. Purine Metabolism: Interconversions 9. Purine Metabolism: Intervention of the pentose phosphate pathway and glycolysis 10. Purine Metabolism: Metabolic consensus 11. Pyrimidine Metabolism: Interconversions and metabolic consensus 12. Predicting Metabolism 13. Acknowledgements 14. References 1. ABSTRACT Bacteria of the class Mollicutes have no cell wall. represent these simple microbes. Mycoplasma genitalium, a One species, Mycoplasma genitalium is the personification Mollicutes with a genome of 580 kbp and 475 ORFs, has of the simplest form of independent cell-free life. Its small the smallest genome in any free-living cell and is an genome (580 kbp) is the smallest of any cell. Mollicutes obvious example of the simplest organism. It is the minimal have unique metabolic properties, perhaps because of their cell and defines-characterizes, personifies independent limited coding space and high mutability. Based on 16S cellular life. rRNA analyses the Mollicutes Mycoplasma gallisepticum is thought to be the most mutable Bacteria. Enzyme activities All Mollicutes, like M. -
Generate Metabolic Map Poster
Authors: Zheng Zhao, Delft University of Technology Marcel A. van den Broek, Delft University of Technology S. Aljoscha Wahl, Delft University of Technology Wilbert H. Heijne, DSM Biotechnology Center Roel A. Bovenberg, DSM Biotechnology Center Joseph J. Heijnen, Delft University of Technology An online version of this diagram is available at BioCyc.org. Biosynthetic pathways are positioned in the left of the cytoplasm, degradative pathways on the right, and reactions not assigned to any pathway are in the far right of the cytoplasm. Transporters and membrane proteins are shown on the membrane. Marco A. van den Berg, DSM Biotechnology Center Peter J.T. Verheijen, Delft University of Technology Periplasmic (where appropriate) and extracellular reactions and proteins may also be shown. Pathways are colored according to their cellular function. PchrCyc: Penicillium rubens Wisconsin 54-1255 Cellular Overview Connections between pathways are omitted for legibility. Liang Wu, DSM Biotechnology Center Walter M. van Gulik, Delft University of Technology L-quinate phosphate a sugar a sugar a sugar a sugar multidrug multidrug a dicarboxylate phosphate a proteinogenic 2+ 2+ + met met nicotinate Mg Mg a cation a cation K + L-fucose L-fucose L-quinate L-quinate L-quinate ammonium UDP ammonium ammonium H O pro met amino acid a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar a sugar K oxaloacetate L-carnitine L-carnitine L-carnitine 2 phosphate quinic acid brain-specific hypothetical hypothetical hypothetical hypothetical -

O O2 Enzymes Available from Sigma Enzymes Available from Sigma
COO 2.7.1.15 Ribokinase OXIDOREDUCTASES CONH2 COO 2.7.1.16 Ribulokinase 1.1.1.1 Alcohol dehydrogenase BLOOD GROUP + O O + O O 1.1.1.3 Homoserine dehydrogenase HYALURONIC ACID DERMATAN ALGINATES O-ANTIGENS STARCH GLYCOGEN CH COO N COO 2.7.1.17 Xylulokinase P GLYCOPROTEINS SUBSTANCES 2 OH N + COO 1.1.1.8 Glycerol-3-phosphate dehydrogenase Ribose -O - P - O - P - O- Adenosine(P) Ribose - O - P - O - P - O -Adenosine NICOTINATE 2.7.1.19 Phosphoribulokinase GANGLIOSIDES PEPTIDO- CH OH CH OH N 1 + COO 1.1.1.9 D-Xylulose reductase 2 2 NH .2.1 2.7.1.24 Dephospho-CoA kinase O CHITIN CHONDROITIN PECTIN INULIN CELLULOSE O O NH O O O O Ribose- P 2.4 N N RP 1.1.1.10 l-Xylulose reductase MUCINS GLYCAN 6.3.5.1 2.7.7.18 2.7.1.25 Adenylylsulfate kinase CH2OH HO Indoleacetate Indoxyl + 1.1.1.14 l-Iditol dehydrogenase L O O O Desamino-NAD Nicotinate- Quinolinate- A 2.7.1.28 Triokinase O O 1.1.1.132 HO (Auxin) NAD(P) 6.3.1.5 2.4.2.19 1.1.1.19 Glucuronate reductase CHOH - 2.4.1.68 CH3 OH OH OH nucleotide 2.7.1.30 Glycerol kinase Y - COO nucleotide 2.7.1.31 Glycerate kinase 1.1.1.21 Aldehyde reductase AcNH CHOH COO 6.3.2.7-10 2.4.1.69 O 1.2.3.7 2.4.2.19 R OPPT OH OH + 1.1.1.22 UDPglucose dehydrogenase 2.4.99.7 HO O OPPU HO 2.7.1.32 Choline kinase S CH2OH 6.3.2.13 OH OPPU CH HO CH2CH(NH3)COO HO CH CH NH HO CH2CH2NHCOCH3 CH O CH CH NHCOCH COO 1.1.1.23 Histidinol dehydrogenase OPC 2.4.1.17 3 2.4.1.29 CH CHO 2 2 2 3 2 2 3 O 2.7.1.33 Pantothenate kinase CH3CH NHAC OH OH OH LACTOSE 2 COO 1.1.1.25 Shikimate dehydrogenase A HO HO OPPG CH OH 2.7.1.34 Pantetheine kinase UDP- TDP-Rhamnose 2 NH NH NH NH N M 2.7.1.36 Mevalonate kinase 1.1.1.27 Lactate dehydrogenase HO COO- GDP- 2.4.1.21 O NH NH 4.1.1.28 2.3.1.5 2.1.1.4 1.1.1.29 Glycerate dehydrogenase C UDP-N-Ac-Muramate Iduronate OH 2.4.1.1 2.4.1.11 HO 5-Hydroxy- 5-Hydroxytryptamine N-Acetyl-serotonin N-Acetyl-5-O-methyl-serotonin Quinolinate 2.7.1.39 Homoserine kinase Mannuronate CH3 etc. -
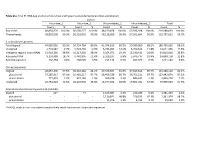
Table S1a. Trial #1 RNA-Seq Analysis of Virus-Free and Hypovirus
Table S1a. Trial #1 RNA-Seq analysis of virus-free and hypovirus-transfected Sclerotinia sclerotiorum Library Virus-free_1 Virus-free_2 Virus-infected_1 Virus-infected_2 Total Count % Count % Count % Count % Count % Raw reads 48,854,170 100.0% 56,568,277 100.0% 48,169,458 100.0% 57,396,928 100.0% 210,988,833 100.0% Filtered reads 48,810,008 99.9% 56,518,010 99.9% 48,118,009 99.9% 57,341,604 99.9% 210,787,631 99.9% S. sclerotiorum genome Total aligned 44,085,026 90.3% 50,724,764 89.8% 41,374,916 86.0% 50,580,960 88.2% 186,765,666 88.6% Unaligned 4,724,982 9.7% 5,793,246 10.3% 6,743,093 14.0% 6,760,644 11.8% 24,021,965 11.5% Intergenic regions (non-rRNA) 13,918,285 28.5% 16,317,039 28.9% 9,354,071 19.4% 15,230,421 26.6% 54,819,816 25.8% Ribosomal RNA 9,150,409 18.7% 6,442,905 11.4% 3,329,107 6.8% 5,976,737 10.4% 24,899,158 11.8% Retrotransposons 202,264 0.4% 268,505 0.5% 251,134 0.5% 499,377 0.9% 1,221,280 0.6% Coding sequences Aligned 28,057,390 57.5% 36,310,362 64.2% 29,370,507 61.0% 37,649,843 65.7% 131,388,102 62.1% plus strand 27,285,917 97.3% 35,481,217 97.7% 28,409,529 96.7% 36,766,211 97.7% 127,942,874 97.3% minus strand 771,473 2.7% 829,145 2.3% 960,978 3.3% 883,632 2.3% 3,445,228 2.7% Unaligned 20,752,618 42.5% 20,207,648 35.8% 18,747,502 39.0% 19,691,761 34.3% 79,399,529 37.9% Sclerotinia sclerotiorum hypovirus 2L (SsHV2L) Aligned 62* - 77 - 1,165,087 2.4% 216,286 0.4% 1,381,450 1.4% plus strand - - - - 1,150,841 98.8% 210,533 97.3% 1,361,374 98.1% minus strand - - - - 14,246 1.2% 5,753 2.7% 19,999 1.9% *SsHV2L reads in non-inoculated samples likely result from errors in barcode sequences.