Seizures in Encephalitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Progressive Multifocal Leukoencephalopathy and the Spectrum of JC Virus-Related Disease
REVIEWS Progressive multifocal leukoencephalopathy and the spectrum of JC virus- related disease Irene Cortese 1 ✉ , Daniel S. Reich 2 and Avindra Nath3 Abstract | Progressive multifocal leukoencephalopathy (PML) is a devastating CNS infection caused by JC virus (JCV), a polyomavirus that commonly establishes persistent, asymptomatic infection in the general population. Emerging evidence that PML can be ameliorated with novel immunotherapeutic approaches calls for reassessment of PML pathophysiology and clinical course. PML results from JCV reactivation in the setting of impaired cellular immunity, and no antiviral therapies are available, so survival depends on reversal of the underlying immunosuppression. Antiretroviral therapies greatly reduce the risk of HIV-related PML, but many modern treatments for cancers, organ transplantation and chronic inflammatory disease cause immunosuppression that can be difficult to reverse. These treatments — most notably natalizumab for multiple sclerosis — have led to a surge of iatrogenic PML. The spectrum of presentations of JCV- related disease has evolved over time and may challenge current diagnostic criteria. Immunotherapeutic interventions, such as use of checkpoint inhibitors and adoptive T cell transfer, have shown promise but caution is needed in the management of immune reconstitution inflammatory syndrome, an exuberant immune response that can contribute to morbidity and death. Many people who survive PML are left with neurological sequelae and some with persistent, low-level viral replication in the CNS. As the number of people who survive PML increases, this lack of viral clearance could create challenges in the subsequent management of some underlying diseases. Progressive multifocal leukoencephalopathy (PML) is for multiple sclerosis. Taken together, HIV, lymphopro- a rare, debilitating and often fatal disease of the CNS liferative disease and multiple sclerosis account for the caused by JC virus (JCV). -

Encephalitis Fact Sheet
Encephalitis Fact Sheet What is encephalitis? Encephalitis is an inflammation (irritation and swelling) of the brain tissue. It can occur as a primary illness or as a result of another illness elsewhere in the body. Frequently caused by an infection with a virus, encephalitis can be mild or severe, but severe cases are rare. Who gets encephalitis? Anyone can get encephalitis, but young children, older adults, and persons with weakened immune systems are more at risk. How is encephalitis spread? Primary encephalitis occurs when a virus directly infects the brain. Viruses that are carried by infected mosquitoes and ticks can cause primary encephalitis. Other viruses can cause an illness and then encephalitis can develop as a complication of the viral illness. When encephalitis develops after another infection, it is called post-infectious encephalitis. Post-infectious encephalitis most commonly follows a bout of chickenpox, mumps, or measles. What are the symptoms of encephalitis? Encephalitis frequently causes headache, fever, confused thinking, seizures, or problems with vision, speech, or movement. Infants and young children might have nausea, vomiting, body stiffness, constant crying, and poor feeding. How soon after exposure do symptoms appear? Encephalitis can occur within two to three weeks after a viral illness. Symptoms begin within a few days to one or two weeks after the bite from an infected mosquito. How is encephalitis diagnosed? Medical evaluation and special tests are needed to confirm the diagnosis. Anyone with a severe headache, fever, and altered mental status should seek medical care immediately. What is the treatment for encephalitis? Doctors might prescribe antiviral drugs, anti-inflammatory drugs, or other treatments for the symptoms as needed. -
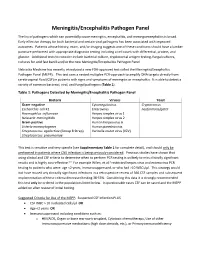
Meningitis/Encephalitis Pathogen Panel
Meningitis/Encephalitis Pathogen Panel The list of pathogens which can potentially cause meningitis, encephalitis, and meningoencephalitis is broad. Early effective therapy for both bacterial and certain viral pathogens has been associated with improved outcomes. Patients whose history, exam, and/or imaging suggests one of these conditions should have a lumber puncture performed with appropriate diagnostic testing including a cell count with differential, protein, and glucose. Additional tests to consider include bacterial culture, cryptococcal antigen testing, fungal cultures, cultures for acid fast bacilli and/or the new Meningitis/Encephalitis Pathogen Panel. Nebraska Medicine has recently introduced a new FDA-approved test called the Meningitis/Encephalitis Pathogen Panel (MEPP). This test uses a nested multiplex PCR-approach to amplify DNA targets directly from cerebrospinal fluid (CSF) in patients with signs and symptoms of meningitis or encephalitis. It is able to detect a variety of common bacterial, viral, and fungal pathogens (Table 1). Table 1: Pathogens Detected by Meningitis/Encephalitis Pathogen Panel Bacteria Viruses Yeast Gram-negative Cytomegalovirus Cryptococcus Escherichia coli K1 Enterovirus neoformans/gattii Haemophilus influenzae Herpes simplex virus 1 Neisseria meningitidis Herpes simplex virus 2 Gram-positive Human herpesvirus 6 Listeria monocytogenes Human parechovirus Streptococcus agalactiae (Group B Strep) Varicella zoster virus (VZV) Streptococcus pneumoniae This test is sensitive and very specific (see Supplementary Table 1 for complete detail), and should only be performed in patients where CNS infection is being seriously considered. Previous studies have shown that using clinical and CSF criteria to determine when to perform PCR testing is unlikely to miss clinically significant results and is highly cost-effective.1-3 For example Wilen, et al.3 restricted herpes virus and enterovirus PCR testing to patients who were: age <2 years, immunosuppressed, or who had >10 WBCs/µl. -

Granulomatous Meningoencephalitis and Necrotizing Encephalitis
Granulomatous Meningoencephalitis and Necrotizing Encephalitis A. Courtenay Freeman, DVM Marc Kent, DVM, DACVIM (Small Animal and Neurology) Scott J. Schatzberg, DVM, PhD, DACVIM (Neurology) BASIC INFORMATION TREATMENT AND FOLLOW-UP Description Granulomatous meningoencephalitis (GME) and necrotizing Treatment Options encephalitis (NE) are disorders that arise from inflammation of the Because GME and NE are caused by inflammation, treatment is brain and/or spinal cord and their coverings (the meninges). These initially directed at suppressing the inflammation with steroids. diseases affect predominantly young, small-breed dogs. High doses are used initially; as the animal improves, the dosage Causes may be lowered gradually over the course of months. The goal Both GME and NE are presently considered to be autoimmune dis- of therapy is to reduce the dosage to the minimal amount needed orders, meaning that the body launches an abnormal attack against to control the clinical signs. Side effects of steroids include its own nervous system tissues. Potential triggers for the autoim- increased thirst, appetite, and urination. Animals may breathe mune attack may include infections and vaccinations. Genetics heavily and pant excessively. Long-term side effects include a may also play a role in the development of GME and NE. thin hair coat, poor wound healing, and muscle loss. Steroid ther- Although any breed of dog may be affected with GME, toy apy can also make the dog prone to infections of the skin and breeds are affected most often. Breeds commonly affected by NE urinary tract. Notify your veterinarian if any of these side effects include the pug, Maltese, Yorkshire terrier, Chihuahua, French occur. -

Review of Case Definitions for Myalgic Encephalomyelitis/Chronic Fatigue
Lim and Son J Transl Med (2020) 18:289 https://doi.org/10.1186/s12967-020-02455-0 Journal of Translational Medicine REVIEW Open Access Review of case defnitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Eun‑Jin Lim and Chang‑Gue Son* Abstract Background: Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a debilitating disease with unknown causes. From the perspectives on the etiology and pathophysiology, ME/CFS has been labeled diferently, which infu‑ enced changes in case defnitions and terminologies. This review sought to feature aspects of the history, develop‑ ments, and diferential symptoms in the case defnitions. Methods: A search was conducted through PubMed published to February 2020 using the following search key‑ words: case defnition AND chronic fatigue syndrome [MeSH Terms]. All reference lists of the included studies were checked. Of the included studies, the number of citations and the visibility in the literatures of the defnitions were considered for comparisons of the criteria. Results: Since the frst ’ME’ case defnition was developed in 1986, 25 case defnitions/diagnostic criteria were cre‑ ated based on three conceptual factors (etiology, pathophysiology, and exclusionary disorders). These factors can be categorized into four categories (ME, ME/CFS, CFS, and SEID) and broadly characterized according to primary disorder (ME‑viral, CFS‑unknown, ME/CFS‑infammatory, SEID‑multisystemic), compulsory symptoms (ME and ME/ CFS‑neuroinfammatory, CFS and SEID‑fatigue and/or malaise), and required conditions (ME‑infective agent, ME/ CFS, CFS, SEID‑symptoms associated with fatigue, e.g., duration of illness). ME and ME/CFS widely cover all symptom categories, while CFS mainly covers neurologic and neurocognitive symptoms. -

Tick-Borne Encephalitis (TBE)
Tick-borne Encephalitis (TBE) Tick-borne encephalitis, or TBE, is a human viral infectious disease involving the central nervous system. TBE is caused by the tick- borne encephalitis virus (TBEV), a member of the family Flaviviridae, and was initially isolated in 1937. Three virus sub-types are described: European or Western tick-borne encephalitis virus, Siberian tick-borne encephalitis virus, and Far eastern Tick-borne encephalitis virus (formerly known as Russian Spring Summer encephalitis virus, RSSEV). The family Flaviviridae includes several tick-borne viruses affecting humans. These viruses are closely related to TBEV and Far- eastern TBE, and include Omsk hemorrhagic fever virus in Siberia, Kyasanur Forest disease virus in India and its close relative, Alkhurma virus in Saudi Arabia. Louping ill virus (United Kingdom) is also a member of this family; it causes disease primarily in sheep and has been reported as the cause of a TBE-like illness in laboratory workers and persons with contact to sick sheep (e.g., veterinarians, butchers). In the USA and Russia, another tick-borne flavivirus, Powassan virus, is responsible of encephalitis in human. Transmission Ticks, specifically hard ticks of the family Ixodidae, act as both the vector and reservoir for TBEV. The main hosts are small rodents, with humans being accidental hosts. Large animals serve as feeding hosts for the ticks, but do not play a role in maintenance of the virus. The virus can chronically infect ticks and is transmitted both transtadially (from larva to nymph to adult ticks) and transovarially (from adult female tick to eggs). TBE cases occur in humans most frequently in rural areas and during the highest period of tick activity (between April and November). -

Chronic Viral Infections in Myalgic Encephalomyelitis/Chronic Fatigue
Rasa et al. J Transl Med (2018) 16:268 https://doi.org/10.1186/s12967-018-1644-y Journal of Translational Medicine REVIEW Open Access Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Santa Rasa1, Zaiga Nora‑Krukle1, Nina Henning2, Eva Eliassen2 , Evelina Shikova4, Thomas Harrer5, Carmen Scheibenbogen6, Modra Murovska1 and Bhupesh K. Prusty2,3* on behalf of the European Network on ME/CFS (EUROMENE) Abstract Background and main text: Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a complex and controversial clinical condition without having established causative factors. Increasing numbers of cases during past decade have created awareness among patients as well as healthcare professionals. Chronic viral infection as a cause of ME/CFS has long been debated. However, lack of large studies involving well-designed patient groups and validated experimental set ups have hindered our knowledge about this disease. Moreover, recent developments regarding molecular mechanism of pathogenesis of various infectious agents cast doubts over validity of several of the past studies. Conclusions: This review aims to compile all the studies done so far to investigate various viral agents that could be associated with ME/CFS. Furthermore, we suggest strategies to better design future studies on the role of viral infec‑ tions in ME/CFS. Keywords: ME/CFS, Viral infections, Biomarkers Background [4]. According to the available literature, already back in Myalgic encephalomyelitis/chronic fatigue syndrome 2009 around 17 million people were diagnosed with this (ME/CFS) is a disease that causes central nervous sys- disease, including 800,000 patients in the United States tem (CNS) and immune system disturbances, cell energy of America and 240,000 in the United Kingdom [5]. -

Basic Fact Sheet - Viral Encephalitis in Animals Texas Department of Health, Zoonosis Control Division
Basic Fact Sheet - Viral Encephalitis in Animals Texas Department of Health, Zoonosis Control Division What is viral encephalitis? Viral encephalitis is a brain infection caused by specific viruses, such as eastern, western and Venezuelan equine encephalitis (EEE, WEE, and VEE) and West Nile virus (WNV). In normal situations, these diseases are transmitted by mosquitoes and are best known for affecting horses and people. Other domestic animals may also be affected by some of these viruses. How can an animal get viral encephalitis? The viruses are spread by mosquitoes. What are the signs of viral encephalitis? After ½ to 2 days, horses will have fever and a fast heart rate; they will stop eating, and look depressed. Weakness and staggering are followed by muscle spasms, chewing movements, incoordination (loss of balance), and seizures. The survival rate (number that live) varies for the different viruses. Of the infected animals that do not survive, some will die rapidly (within a few hours) while others could live several weeks before dying. How is viral encephalitis diagnosed? Only laboratory testing can provide a definite diagnosis. How is viral encephalitis treated? Treatment of animals showing signs of disease is limited to nursing care. Is a viral encephalitis vaccine available? Vaccines are available for horses. They should be given yearly. To be effective, the horse must be vaccinated before it is exposed to a virus. Can infected animals spread viral encephalitis? Mosquitoes spread the disease to horses and people after feeding on infected birds. Mosquitoes cannot spread the viruses from infected horses to other animals and people. What is done with animals that die of viral encephalitis? There are no special burial or disposal requirements. -

Acute Encephalitis Is a Neurological Emergency Which Can Lumbar Puncture (LP), Which in Practice Is Often Delayed
Clinical Medicine 2018 Vol 18, No 2: 155–9 CME INFECTIOUS DISEASES A c u t e e n c e p h a l i t i s – d i a g n o s i s a n d m a n a g e m e n t A u t h o r s : M a r k E l l u l A, B a n d T o m S o l o m o n C, D Encephalitis, infl ammation of the brain, is most commonly Box 1. Definitions (as used in research studies) 4 caused by a viral infection (especially herpes simplex virus = [HSV] type 1 in the UK) although autoimmune causes, such as Encephalopathy (altered consciousness persisting for N-methyl D-aspartate receptor (NMDAR) antibody encepha- longer than 24 h, including lethargy, irritability or a change in litis, are increasingly recognised. Most patients present with personality or behaviour) ABSTRACT a change in consciousness level and may have fever, seizures, Encephalitis = encephalopathy AND evidence of CNS movement disorder or focal neurological defi cits. Diagnosis inflammation, demonstrated by at least two of: hinges crucially on lumbar puncture and cerebrospinal fl uid > fever (CSF) examination, but imaging and electroencephalography seizures or focal neurological findings attributable to the (EEG) may also be helpful. Treatment of HSV encephalitis with > brain parenchyma aciclovir dramatically improves outcome, but the optimal man- agement of autoimmune encephalitis is still uncertain. Many > CSF pleocytosis (more than 4 white cells per μL) patients with encephalitis are left with residual physical or > EEG findings suggestive of encephalitis neuropsychological defi cits which require long-term multidis- neuroimaging findings suggestive of encephalitis. -

Spontaneous Recovery from Progressive Multifocal Leukoencephalopathy in a Patient with Non-Active Sarcoidosis
International Journal of Infectious Diseases 14S (2010) e313–e316 Contents lists available at ScienceDirect International Journal of Infectious Diseases journal homepage: www.elsevier.com/locate/ijid Case Report Spontaneous recovery from progressive multifocal leukoencephalopathy in a patient with non-active sarcoidosis Annemarie Goldbecker a,*, Argyro Tountopoulou a, Ulrich Wurster a, Frank Donnerstag b, Almuth Brandis c, Catharina Bonnemann a, Karin Weissenborn a a Department of Neurology and Neurophysiology, Hannover Medical School, Carl-Neuberg Straße 1, 30625 Hannover, Germany b Institute for Diagnostic and Interventional Neuroradiology, Hannover Medical School, Hannover, Germany c Institute for Pathology, Hannover Medical School, Hannover, Germany ARTICLE INFO SUMMARY Article history: We report the case of a 50-year-old female patient with non-active sarcoidosis and no kind of Received 24 February 2009 immunosuppression, admitted to our hospital because of increasing confusion and focal neurological Received in revised form 3 October 2009 deficits. Initially a tumor, herpes encephalitis, or neurosarcoidosis were suspected, but surprisingly Accepted 25 February 2010 biopsy revealed progressive multifocal leukoencephalopathy, additionally confirmed by JC-positive PCR Corresponding Editor: William Cameron, in cerebrospinal fluid. Cases of sarcoidosis and progressive multifocal leukoencephalopathy have been Ottawa, Canada reported before. This is the first case of a patient with no sign of active sarcoidosis and without immunosuppressive therapy who recovered spontaneously with a follow-up time of nearly 3 years. Keywords: ß 2010 International Society for Infectious Diseases. Published by Elsevier Ltd. All rights reserved. Progressive multifocal leukoencephalopathy Sarcoidosis Magnetic resonance imaging 1. Introduction methotrexate had been given from 1992 to 1993 and occasional therapy with corticosteroids between 1988 and 1997. -

Fifty Years of JC Polyomavirus: a Brief Overview and Remaining Questions
viruses Review Fifty Years of JC Polyomavirus: A Brief Overview and Remaining Questions Abigail L. Atkinson and Walter J. Atwood * Department of Molecular Biology, Cell Biology and Biochemistry, Brown University, Providence, RI 02912, USA; [email protected] * Correspondence: [email protected] Received: 25 August 2020; Accepted: 30 August 2020; Published: 1 September 2020 Abstract: In the fifty years since the discovery of JC polyomavirus (JCPyV), the body of research representing our collective knowledge on this virus has grown substantially. As the causative agent of progressive multifocal leukoencephalopathy (PML), an often fatal central nervous system disease, JCPyV remains enigmatic in its ability to live a dual lifestyle. In most individuals, JCPyV reproduces benignly in renal tissues, but in a subset of immunocompromised individuals, JCPyV undergoes rearrangement and begins lytic infection of the central nervous system, subsequently becoming highly debilitating—and in many cases, deadly. Understanding the mechanisms allowing this process to occur is vital to the development of new and more effective diagnosis and treatment options for those at risk of developing PML. Here, we discuss the current state of affairs with regards to JCPyV and PML; first summarizing the history of PML as a disease and then discussing current treatment options and the viral biology of JCPyV as we understand it. We highlight the foundational research published in recent years on PML and JCPyV and attempt to outline which next steps are most necessary to reduce the disease burden of PML in populations at risk. Keywords: progressive multifocal leukoencephalopathy; JC polyomavirus; polyomavirus; HIV/AIDS; multiple sclerosis; autoimmune disease 1. -

Infectious Meningitis and Encephalitis
367 Infectious Meningitis and Encephalitis Amanda L. Piquet, MD1 Jennifer L. Lyons, MD2,3 1 Division of Neuroimmunology, Department of Neurology, University Address for correspondence Jennifer L. Lyons, MD, Division of of Utah Hospital, Salt Lake City, Utah Neurological Infections and Inflammatory Diseases, Department of 2 Division of Neurological Infections and Inflammatory Diseases, Neurology, Brigham and Women’s Hospital, Boston, MA 02115 Department of Neurology, Brigham and Women’sHospital, (e-mail: [email protected]). Boston, Massachusetts 3 Harvard Medical School, Boston, Massachusetts Semin Neurol 2016;36:367–372. Abstract The clinician who is evaluating a patient with a suspected central nervous system infection often faces a large differential diagnosis. There are several signs, symptoms, geographical clues, and diagnostic testing, such as cerebrospinal fluid abnormalities Keywords and magnetic resonance imaging abnormalities, which can be helpful in identifying the ► encephalitis etiological agent. By taking a systematic approach, one can often identify life- ► meningitis threatening, common, and/or treatable etiologies. Here the authors describe some ► herpes simplex virus of the pearls and pitfalls in diagnosing and treating acute infectious meningitis and ► arbovirus encephalitis. Encephalitis concomitant use of corticosteroids in the setting of properly treating the infection. This is discussed further in the Treat- Clinical Presentation ment section below. It is important to identify the clinical differences between Herpes simplex virus (HSV) is the most commonly identi- encephalitis and encephalopathy. Encephalitis is a pathologic fied etiology of viral encephalitis in developed countries. diagnosis, but the International Encephalitis Consortium1 has Ninety percent of herpes simplex virus encephalitis (HSVE) defined clinical criteria to assist with bedside diagnosis.