Autoimmune Encephalitis and Epilepsy
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Spontaneous Spinal Cerebrospinal Fluid Leaks and Intracranial Hypotension
CLINICAL REVIEW CLINICIAN’S CORNER Spontaneous Spinal Cerebrospinal Fluid Leaks and Intracranial Hypotension Wouter I. Schievink, MD Context Spontaneous intracranial hypotension is caused by spontaneous spinal ce- PATIENT PRESENTS WITH A rebrospinal fluid (CSF) leaks and is known for causing orthostatic headaches. It is an new headache that occurs important cause of new headaches in young and middle-aged individuals, but initial shortly after assuming an up- misdiagnosis is common. right position and is re- Objective To summarize existing evidence regarding the epidemiology, pathophysi- Alieved by lying down. Although such a ology, diagnosis, and management of spontaneous spinal CSF leaks and intracranial positional headache pattern is well- hypotension. known following a diagnostic lumbar Evidence Acquisition MEDLINE (1966-2005) and OLDMEDLINE (1950-1965) were puncture, the spontaneous onset of an searched using the terms intracranial hypotension, CSF leak, low pressure headache, orthostatic headache is not well recog- and CSF hypovolemia. Reference lists of these articles and ongoing investigations in nized and the patient may be diag- this area were used as well. nosed with migraine, tension head- Evidence Synthesis Spontaneous intracranial hypotension is caused by single or ache, viral meningitis, or malingering. multiple spinal CSF leaks. The incidence has been estimated at 5 per 100 000 per year, This has been a typical scenario for with a peak around age 40 years. Women are affected more commonly than men. Mechanical factors combine with an underlying connective tissue disorder to cause many patients experiencing spontane- 1 the CSF leaks. An orthostatic headache is the prototypical manifestation but other head- ous intracranial hypotension. -

Pseudomigraine with Pleocytosis
P1: KWW/KKL P2: KWW/HCN QC: KWW/FLX T1: KWW GRBT050-115 Olesen- 2057G GRBT050-Olesen-v6.cls August 17, 2005 1:50 ••Chapter 115 ◗ Pseudomigraine with Pleocytosis Dimos D. Mitsikostas and Julio Pascual Other terms: migraine variants, headache and neurologic dle cerebral artery (9). Alternatively, imaging studies sug- deficits with cerebrospinal fluid lymphocytosis (HaNDL) gest that neurologic deficits of HaDNL might be caused by cortical spreading depression (5,6), as in migraine with aura. Furthermore, overlap of clinical presentations be- INTRODUCTION tween HaNDL and familial hemiplegic migraine triggered a search for mutations in the CACNA1A gene, which were Pseudomigraine is a term first introduced in 1983 to not found (4). Finally, the CSF inflammatory picture of describe attacks of temporary neurologic deficit accom- HaNDL suggests an inflammatory, autoimmune, or postin- panied or followed by migrainous headache and cere- fectious etiology, and supportive data are awaited. brospinal fluid (CSF) pleocytosis of unknown origin and automatic relief (10). Similar clinical presentations were reported previously under different names, such as mi- PRESENTATION AND EVALUATION graine variants (1,13). Diagnostic criteria (Revised International Classification for Headache Disorders [ICHD-II]) of HaNDL are listed EPIDEMIOLOGY in Table 115-1. The clinical characteristics of HaNDL are: (1) more than two discrete episodes of temporal focal Fewer than 100 cases of HaNDL are reported in the liter- neurologic (most often sensory plus aphasic) symptoms ature (2,7), but it may be underdiagnosed as estimates of followed by moderate to severe headache and (2) com- its annual incidence are up to 0.2 cases per 100,000 inhab- plete and spontaneous relief of the condition after a few itants (11). -

Syringomyelia in Cervical Spondylosis: a Rare Sequel H
THIEME Editorial 1 Editorial Syringomyelia in Cervical Spondylosis: A Rare Sequel H. S. Bhatoe1 1 Department of Neurosciences, Max Super Specialty Hospital, Patparganj, New Delhi, India Indian J Neurosurg 2016;5:1–2. Neurological involvement in cervical spondylosis usually the buckled hypertrophic ligament flavum compresses the implies radiculopathy or myelopathy. Cervical spondylotic cord. Ischemia due to compromise of microcirculation and myelopathy is the commonest cause of myelopathy in the venous congestion, leading to focal demyelination.3 geriatric age group,1 and often an accompaniment in adult Syringomyelia is an extremely rare sequel of chronic cervical patients manifesting central cord syndrome and spinal cord cord compression due to spondylotic process, and manifests as injury without radiographic abnormality. Myelopathy is the accelerated myelopathy (►Fig. 1). Pathogenesis of result of three factors that often overlap: mechanical factors, syringomyelia is uncertain. Al-Mefty et al4 postulated dynamic-repeated microtrauma, and ischemia of spinal cord occurrence of myelomalacia due to chronic compression of microcirculation.2 Age-related mechanical changes include the cord, followed by phagocytosis, leading to a formation of hypertrophy of the ligamentum flavum, formation of the cavity that extends further. However, Kimura et al5 osteophytic bars, degenerative disc prolapse, all of them disagreed with this hypothesis, and postulated that following contributing to a narrowing of the spinal canal. Degenerative compression of the cord, there is slosh effect cranially and kyphosis and subluxation often aggravates the existing caudally, leading to an extension of the syrinx. It is thus likely compressiononthespinalcord.Flexion–extension that focal cord cavitation due to compression and ischemia movements of the spinal cord places additional, dynamic occurs due to periventricular fluid egress into the cord, the stretch on the cord that is compressed. -

Research Experiences Research Is an Important Part of the Training of Child Neurology Residents at Children’S Mercy Hospital
Research Experiences Research is an important part of the training of Child Neurology residents at Children’s Mercy Hospital. Training in research starts with the research mentor that each resident is encouraged to engage at the beginning of their child neurology training. The residents are also invited to complete a course on biostatistics and each resident is expected to complete a 1 year course in Quality Improvement and Clinical Safety. As part of the QI course each resident will initiate a QI project which can be presented at CMH Research Day. Each resident is also given the opportunity to present at the yearly Missouri Valley Child Neurology Colloquium. This is a joint meeting with The University of Washington and Saint Louis University Child Neurology programs. Finally, each resident is expected to graduate with at least one first author publication. Over the last 4 years our residents have given over 100 talks (with approximately 20% of these original research or case presentations) and have published 13 papers in peer reviewed journals (including original research and review papers). Faculty Program Director Jean-Baptiste (J.B.) Le Pichon, MD, PhD: Dr. Le Pichon was born in New York City but grew up in France. He completed his undergraduate education at Gannon University, Erie Pennsylvania followed by an MD/PhD program at Baylor College of Medicine, Houston Texas. Dr. Le Pichon completed his PhD in neuroscience. Following medical school, he completed two years of Pediatrics at Driscoll Children’s Hospital in Corpus Christi and then completed a Child Neurology Residency at Texas Children’s Hospital. -

Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, and Natural History
Journal of Clinical Medicine Review Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, and Natural History Melissa Lannon and Edward Kachur * Division of Neurosurgery, McMaster University, Hamilton, ON L8S 4L8, Canada; [email protected] * Correspondence: [email protected] Abstract: Degenerative cervical myelopathy (DCM) is a leading cause of spinal cord injury and a major contributor to morbidity resulting from narrowing of the spinal canal due to osteoarthritic changes. This narrowing produces chronic spinal cord compression and neurologic disability with a variety of symptoms ranging from mild numbness in the upper extremities to quadriparesis and incontinence. Clinicians from all specialties should be familiar with the early signs and symptoms of this prevalent condition to prevent gradual neurologic compromise through surgical consultation, where appropriate. The purpose of this review is to familiarize medical practitioners with the pathophysiology, common presentations, diagnosis, and management (conservative and surgical) for DCM to develop informed discussions with patients and recognize those in need of early surgical referral to prevent severe neurologic deterioration. Keywords: degenerative cervical myelopathy; cervical spondylotic myelopathy; cervical decompres- sion Citation: Lannon, M.; Kachur, E. Degenerative Cervical Myelopathy: Clinical Presentation, Assessment, 1. Introduction and Natural History. J. Clin. Med. Degenerative cervical myelopathy (DCM) is now the leading cause of spinal cord in- 2021, 10, 3626. https://doi.org/ jury [1,2], resulting in major disability and reduced quality of life. While precise prevalence 10.3390/jcm10163626 is not well described, a 2017 Canadian study estimated a prevalence of 1120 per million [3]. DCM results from narrowing of the spinal canal due to osteoarthritic changes. This Academic Editors: Allan R. -

A Histopathological and Immunohistochemical Study of Acute and Chronic Human Compressive Myelopathy
Cellular Pathology and Apoptosis in Experimental and Human Acute and Chronic Compressive Myelopathy ROWENA ELIZABETH ANNE NEWCOMBE M.B.B.S. B.Med Sci. (Hons.) Discipline of Pathology, School of Medical Sciences University of Adelaide June 2010 A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy CHAPTER 1 INTRODUCTION 1 The term “compressive myelopathy” describes a spectrum of spinal cord injury secondary to compressive forces of varying magnitude and duration. The compressive forces may act over a short period of time, continuously, intermittently or in varied combination and depending on their magnitude may produce a spectrum varying from mild to severe injury. In humans, spinal cord compression may be due to various causes including sudden fracture/dislocation and subluxation of the vertebral column, chronic spondylosis, disc herniation and various neoplasms involving the vertebral column and spinal canal. Neoplasms may impinge on the spinal cord and arise from extramedullary or intramedullary sites. Intramedullary expansion producing a type of internal compression can be due to masses created by neoplasms or fluid such as the cystic cavitation seen in syringomyelia. Acute compression involves an immediate compression of the spinal cord from lesions such as direct trauma. Chronic compression may develop over weeks to months or years from conditions such as cervical spondylosis which may involve osteophytosis or hypertrophy of the adjacent ligamentum flavum. Compressive myelopathies include the pathological changes from direct mechanical compression at one or multiple levels and changes in the cord extending multiple segments above and below the site of compression. Evidence over the past decade suggests that apoptotic cell death in neurons and glia, in particular of oligodendrocytes, may play an important role in the pathophysiology and functional outcome of human chronic compressive myelopathy. -

Seizures in Encephalitis
Neurology Asia 2008; 13 : 1 – 13 REVIEW ARTICLES Seizures in encephalitis Usha Kant Misra DM, *C T Tan MD, Jayantee Kalita DM Department of Neurology, Sanjay Gandhi PGIMS, Lucknow, India; *Department of Medicine, University of Malaya, Kuala Lumpur, Malaysia Abstract A large number of viruses can result in encephalitis. However, certain viruses are more prevalent in certain geographical regions. For example, Japanese encephalitis (JE) and dengue in South East Asia and West Nile in Middle East whereas Herpes simplex encephalitis (HSE) occurs all over the world without any seasonal or regional variation. Encephalitis can result in acute symptomatic seizures and remote symptomatic epilepsy. Risk of seizures after 20 years is 22% following encephalitis and 3% after meningitis with early seizures. Amongst the viruses, HSE is associated with most frequent and severe epilepsy. Seizures may be presenting feature in 50% because of involvement of highly epileptogenic frontotemporal cortex. Presence of seizures in HSE is associated with poor prognosis. HSE can also result in chronic and relapsing form of encephalitis and may be an aetiology factor in drug resistant epilepsy. Amongst the Flaviviruses, Japanese encephalitis is the most common and is associated with seizures especially in children. The frequency of seizures in JE is reported to be 6.9% to 46%. Associated neurocysticercosis in JE patients may aggravate the frequency and severity of seizures. Other flaviviruses such as equine, St Louis, and West Nile encephalitis can also produce seizures. In Nipah encephalitis, seizures are commoner in relapsed and late-onset encephalitis as compared to acute encephalitis (50% vs 24%). Other viruses like measles, varicella, mumps, influenza and enteroviruses may result in encephalitis and seizures. -

Myoclonus Aspen Summer 2020
Hallett Myoclonus Aspen Summer 2020 Myoclonus (Chapter 20) Aspen 2020 1 Myoclonus: Definition Quick muscle jerks Either irregular or rhythmic, but always simple 2 1 Hallett Myoclonus Aspen Summer 2020 Myoclonus • Spontaneous • Action myoclonus: activated or accentuated by voluntary movement • Reflex myoclonus: activated or accentuated by sensory stimulation 3 Myoclonus • Focal: involving only few adjacent muscles • Generalized: involving most or many of the muscles of the body • Multifocal: involving many muscles, but in different jerks 4 2 Hallett Myoclonus Aspen Summer 2020 Differential diagnosis of myoclonus • Simple tics • Some components of chorea • Tremor • Peripheral disorders – Fasciculation – Myokymia – Hemifacial spasm 9 Classification of Myoclonus Site of Origin • Cortex – Cortical myoclonus, epilepsia partialis continua, cortical tremor • Brainstem – Reticular myoclonus, exaggerated startle, palatal myoclonus • Spinal cord – Segmental, propriospinal • Peripheral – Rare, likely due to secondary CNS changes 10 3 Hallett Myoclonus Aspen Summer 2020 Classification of myoclonus to guide therapy • First consideration: Etiological classification – Is there a metabolic encephalopathy to be treated? Is there a tumor to be removed? Is a drug responsible? • Second consideration: Physiological classification – Can the myoclonus be treated symptomatically even if the underlying condition remains unchanged? 12 Myoclonus: Physiological Classification • Epileptic • Non‐epileptic The basic question to ask is whether the myoclonus is a “fragment -

Progressive Multifocal Leukoencephalopathy and the Spectrum of JC Virus-Related Disease
REVIEWS Progressive multifocal leukoencephalopathy and the spectrum of JC virus- related disease Irene Cortese 1 ✉ , Daniel S. Reich 2 and Avindra Nath3 Abstract | Progressive multifocal leukoencephalopathy (PML) is a devastating CNS infection caused by JC virus (JCV), a polyomavirus that commonly establishes persistent, asymptomatic infection in the general population. Emerging evidence that PML can be ameliorated with novel immunotherapeutic approaches calls for reassessment of PML pathophysiology and clinical course. PML results from JCV reactivation in the setting of impaired cellular immunity, and no antiviral therapies are available, so survival depends on reversal of the underlying immunosuppression. Antiretroviral therapies greatly reduce the risk of HIV-related PML, but many modern treatments for cancers, organ transplantation and chronic inflammatory disease cause immunosuppression that can be difficult to reverse. These treatments — most notably natalizumab for multiple sclerosis — have led to a surge of iatrogenic PML. The spectrum of presentations of JCV- related disease has evolved over time and may challenge current diagnostic criteria. Immunotherapeutic interventions, such as use of checkpoint inhibitors and adoptive T cell transfer, have shown promise but caution is needed in the management of immune reconstitution inflammatory syndrome, an exuberant immune response that can contribute to morbidity and death. Many people who survive PML are left with neurological sequelae and some with persistent, low-level viral replication in the CNS. As the number of people who survive PML increases, this lack of viral clearance could create challenges in the subsequent management of some underlying diseases. Progressive multifocal leukoencephalopathy (PML) is for multiple sclerosis. Taken together, HIV, lymphopro- a rare, debilitating and often fatal disease of the CNS liferative disease and multiple sclerosis account for the caused by JC virus (JCV). -

HIRSCH New When Neurology and Psychology Collide Inflammation V2
WHEN NEUROLOGY & PSYCHIATRY COLLIDE: INFLAMMATION Scott E. Hirsch, MD Clinical Associate Professor NYU School of Medicine March 2018 Disclosures • No Financial Disclosures • There will be discussion of pharmaceutical products being used for non-FDA approved indications !2 What are Tics? A sudden, rapid, recurrent, non-rhythmic motor movement or vocalization. – A tic repertoire may affect any muscle group. – Any repetitive vocalization can be a tic, from throat clearing to sentences. – Tics are involuntary, but in some individuals tics can be voluntarily suppressed . – Suppressing tics typically leads to a buildup of tension. There is also an “urge” to tic. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition, 2013 !3 Tics: Neuroscience • Tics are thought to be due to dysfunction of the Basal Ganglia – Cerebellar – Thalamo - Cortical system. • Reduced cortico-thalamic control of the basal ganglia leads disinhibition of thalamo-cortical feedback. • Leads to extra movements - > motor tics • Leads to extra vocalization -> vocal tics Caligiore D, Mannella F, Arbib MA, Baldassarre G. Dysfunctions of the basal ganglia-cerebellar-thalamo-cortical system produce motor tics in Tourette syndrome. PLoS Comput Biol. 2017 Mar 30;13(3):e1005395. doi: 10.1371/journal.pcbi.1005395 DSM V Diagnostic Criteria for Tourette’s Disorder 307.23 (F95.2) A. Both multiple motor and one or more vocal tics have been present at some time during the illness, although not necessarily concurrently. B. The tics may wax and wane in frequency but have persisted for more than 1 year since first tic onset. C. Onset is before age 18 years. D. The disturbance is not attributable to the physiological effects of a substance (e.g., cocaine) or another medical condition (e.g., Huntington’s disease, post viral encephalitis). -

Encephalitis Fact Sheet
Encephalitis Fact Sheet What is encephalitis? Encephalitis is an inflammation (irritation and swelling) of the brain tissue. It can occur as a primary illness or as a result of another illness elsewhere in the body. Frequently caused by an infection with a virus, encephalitis can be mild or severe, but severe cases are rare. Who gets encephalitis? Anyone can get encephalitis, but young children, older adults, and persons with weakened immune systems are more at risk. How is encephalitis spread? Primary encephalitis occurs when a virus directly infects the brain. Viruses that are carried by infected mosquitoes and ticks can cause primary encephalitis. Other viruses can cause an illness and then encephalitis can develop as a complication of the viral illness. When encephalitis develops after another infection, it is called post-infectious encephalitis. Post-infectious encephalitis most commonly follows a bout of chickenpox, mumps, or measles. What are the symptoms of encephalitis? Encephalitis frequently causes headache, fever, confused thinking, seizures, or problems with vision, speech, or movement. Infants and young children might have nausea, vomiting, body stiffness, constant crying, and poor feeding. How soon after exposure do symptoms appear? Encephalitis can occur within two to three weeks after a viral illness. Symptoms begin within a few days to one or two weeks after the bite from an infected mosquito. How is encephalitis diagnosed? Medical evaluation and special tests are needed to confirm the diagnosis. Anyone with a severe headache, fever, and altered mental status should seek medical care immediately. What is the treatment for encephalitis? Doctors might prescribe antiviral drugs, anti-inflammatory drugs, or other treatments for the symptoms as needed. -
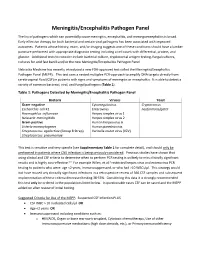
Meningitis/Encephalitis Pathogen Panel
Meningitis/Encephalitis Pathogen Panel The list of pathogens which can potentially cause meningitis, encephalitis, and meningoencephalitis is broad. Early effective therapy for both bacterial and certain viral pathogens has been associated with improved outcomes. Patients whose history, exam, and/or imaging suggests one of these conditions should have a lumber puncture performed with appropriate diagnostic testing including a cell count with differential, protein, and glucose. Additional tests to consider include bacterial culture, cryptococcal antigen testing, fungal cultures, cultures for acid fast bacilli and/or the new Meningitis/Encephalitis Pathogen Panel. Nebraska Medicine has recently introduced a new FDA-approved test called the Meningitis/Encephalitis Pathogen Panel (MEPP). This test uses a nested multiplex PCR-approach to amplify DNA targets directly from cerebrospinal fluid (CSF) in patients with signs and symptoms of meningitis or encephalitis. It is able to detect a variety of common bacterial, viral, and fungal pathogens (Table 1). Table 1: Pathogens Detected by Meningitis/Encephalitis Pathogen Panel Bacteria Viruses Yeast Gram-negative Cytomegalovirus Cryptococcus Escherichia coli K1 Enterovirus neoformans/gattii Haemophilus influenzae Herpes simplex virus 1 Neisseria meningitidis Herpes simplex virus 2 Gram-positive Human herpesvirus 6 Listeria monocytogenes Human parechovirus Streptococcus agalactiae (Group B Strep) Varicella zoster virus (VZV) Streptococcus pneumoniae This test is sensitive and very specific (see Supplementary Table 1 for complete detail), and should only be performed in patients where CNS infection is being seriously considered. Previous studies have shown that using clinical and CSF criteria to determine when to perform PCR testing is unlikely to miss clinically significant results and is highly cost-effective.1-3 For example Wilen, et al.3 restricted herpes virus and enterovirus PCR testing to patients who were: age <2 years, immunosuppressed, or who had >10 WBCs/µl.