The Influence of Virus Infection on Microglia and Accelerated Brain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chasing the Dragon, a Case of Leukoencephalopathy Associated with Heroin Inhalation
Chasing the dragon, a case of leukoencephalopathy associated with heroin inhalation Daniel Cho MD1, Hani Nazha MD2, Kalin Fisher BS2 Author Affiliations: 1. Charleston Area Medical Center, Charleston, West Virginia 2. West Virginia University, Morgantown, West Virginia The authors have no financial disclosures to declare and no conflicts of interest to report. Corresponding Author: Hani Nazha MD West Virginia University Morgantown, West Virginia Email: [email protected] Abstract Although rare, toxic leukoencephalopathy (TLE) associated with heroin inhalation has been reported. “Chasing the dragon” may lead to progressive spongiform degeneration of the brain and presents with a large range of neuropsychological sequelae. This case is an example of TLE in a middle-aged white male with a history of polysubstance abuse. He presented with a three week history of progressive neuropsychological symptoms, including abulia, bradyphrenia, hyperreflexia, and visual hallucinations. He was initially suspected to have progressive multifocal leukoencephalopathy, however, JCV PCR was negative. MRI showed diffuse abnormal signal in the white matter, extending into the thalami and cerebral peduncles. Brain biopsy was performed, which revealed spongiform degeneration, and a diagnosis of TLE was made. The patient was then transferred to a skilled nursing facility. Clinical suspicion based on a thorough history and clinical exam findings is paramount in recognition of heroin-associated TLE. Although rare, heroin-inhalation TLE continues to be reported. As ‘chasing the dragon’ is gaining popularity among drug users, it is important for clinicians to be able to recognize this disease process. Keywords Opioid, Addiction, Heroin, Leukoencephalopathy Introduction Toxic leukoencephalopathy (TLE) associated with heroin abuse was first described in 1982.1 “Chasing the dragon" is a method of heroin vapor inhalation in which a small amount of heroin powder is placed on aluminum foil, which is then heated by placing a match or lighter underneath. -

Viral Encephalitis: a Hard Nut to Crack
Published online: 02.10.2019 THIEME 98 ViralReview Encephalitis Article Shukla et al. Viral Encephalitis: A Hard Nut to Crack Alka Shukla1 Mayank Gangwar1 Sonam Rastogi1 Gopal Nath1 1Department of Microbiology, Viral Research and Diagnostic Address for correspondence Gopal Nath, MD, PhD, Department Laboratory, Institute of Medical Sciences, Banaras Hindu of Microbiology, Viral Research and Diagnostic Laboratory, Institute University, Varanasi, India of Medical Sciences, Banaras Hindu University, Varanasi, India (e-mail: [email protected]). Ann Natl Acad Med Sci (India) 2019;55:98–109 Abstract Viral encephalitis is inflammation of brain that manifests as neurological complication of viral infections. There are quite a good number of viruses, for example, human her- pes virus, Japanese encephalitis, and enteroviruses that can result in such a dreadful condition. Geographical location, age, gender, immune status, and climatic conditions also contribute to the establishment of this disease in an individual. Clinical signs and symptoms include fever, headache, altered level of consciousness, changed mental status, body ache, seizures, nausea, and vomiting. Effective management of this dis- ease relies on timely diagnosis that in turn depends on apt and suitable investigation Keywords techniques. Traditional investigations have thinned out these days owing to the fact ► encephalitis that advanced molecular technologies have been introduced to the diagnostic field. ► viral infection Treatment of viral encephalitis mainly involves symptomatic relieve from fever, mal- ► pathogenesis aise, myalgia along with measures to reduce viral load in the patient. This review men- ► molecular techniques tions about all the possible aspects of viral encephalitis starting from etiology to the ► management management and preventive measures that include immunization and vector control. -

Seizures in Encephalitis
Neurology Asia 2008; 13 : 1 – 13 REVIEW ARTICLES Seizures in encephalitis Usha Kant Misra DM, *C T Tan MD, Jayantee Kalita DM Department of Neurology, Sanjay Gandhi PGIMS, Lucknow, India; *Department of Medicine, University of Malaya, Kuala Lumpur, Malaysia Abstract A large number of viruses can result in encephalitis. However, certain viruses are more prevalent in certain geographical regions. For example, Japanese encephalitis (JE) and dengue in South East Asia and West Nile in Middle East whereas Herpes simplex encephalitis (HSE) occurs all over the world without any seasonal or regional variation. Encephalitis can result in acute symptomatic seizures and remote symptomatic epilepsy. Risk of seizures after 20 years is 22% following encephalitis and 3% after meningitis with early seizures. Amongst the viruses, HSE is associated with most frequent and severe epilepsy. Seizures may be presenting feature in 50% because of involvement of highly epileptogenic frontotemporal cortex. Presence of seizures in HSE is associated with poor prognosis. HSE can also result in chronic and relapsing form of encephalitis and may be an aetiology factor in drug resistant epilepsy. Amongst the Flaviviruses, Japanese encephalitis is the most common and is associated with seizures especially in children. The frequency of seizures in JE is reported to be 6.9% to 46%. Associated neurocysticercosis in JE patients may aggravate the frequency and severity of seizures. Other flaviviruses such as equine, St Louis, and West Nile encephalitis can also produce seizures. In Nipah encephalitis, seizures are commoner in relapsed and late-onset encephalitis as compared to acute encephalitis (50% vs 24%). Other viruses like measles, varicella, mumps, influenza and enteroviruses may result in encephalitis and seizures. -

Everything You Always Wanted to Know About Rabies Virus ♣♣♣♣♣♣♣♣♣♣♣♣♣♣♣♣♣♣♣♣♣ (But Were Afraid to Ask) Benjamin M
ANNUAL REVIEWS Further Click here to view this article's online features: t%PXOMPBEmHVSFTBT115TMJEFT t/BWJHBUFMJOLFESFGFSFODFT t%PXOMPBEDJUBUJPOT Everything You Always Wanted t&YQMPSFSFMBUFEBSUJDMFT t4FBSDILFZXPSET to Know About Rabies Virus (But Were Afraid to Ask) Benjamin M. Davis,1 Glenn F. Rall,2 and Matthias J. Schnell1,2,3 1Department of Microbiology and Immunology and 3Jefferson Vaccine Center, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, Pennsylvania, 19107; email: [email protected] 2Fox Chase Cancer Center, Philadelphia, Pennsylvania 19111 Annu. Rev. Virol. 2015. 2:451–71 Keywords First published online as a Review in Advance on rabies virus, lyssaviruses, neurotropic virus, neuroinvasive virus, viral June 24, 2015 transport The Annual Review of Virology is online at virology.annualreviews.org Abstract This article’s doi: The cultural impact of rabies, the fatal neurological disease caused by in- 10.1146/annurev-virology-100114-055157 fection with rabies virus, registers throughout recorded history. Although Copyright c 2015 by Annual Reviews. ⃝ rabies has been the subject of large-scale public health interventions, chiefly All rights reserved through vaccination efforts, the disease continues to take the lives of about 40,000–70,000 people per year, roughly 40% of whom are children. Most of Access provided by Thomas Jefferson University on 11/13/15. For personal use only. Annual Review of Virology 2015.2:451-471. Downloaded from www.annualreviews.org these deaths occur in resource-poor countries, where lack of infrastructure prevents timely reporting and postexposure prophylaxis and the ubiquity of domestic and wild animal hosts makes eradication unlikely. Moreover, al- though the disease is rarer than other human infections such as influenza, the prognosis following a bite from a rabid animal is poor: There is cur- rently no effective treatment that will save the life of a symptomatic rabies patient. -

Progressive Multifocal Leukoencephalopathy and the Spectrum of JC Virus-Related Disease
REVIEWS Progressive multifocal leukoencephalopathy and the spectrum of JC virus- related disease Irene Cortese 1 ✉ , Daniel S. Reich 2 and Avindra Nath3 Abstract | Progressive multifocal leukoencephalopathy (PML) is a devastating CNS infection caused by JC virus (JCV), a polyomavirus that commonly establishes persistent, asymptomatic infection in the general population. Emerging evidence that PML can be ameliorated with novel immunotherapeutic approaches calls for reassessment of PML pathophysiology and clinical course. PML results from JCV reactivation in the setting of impaired cellular immunity, and no antiviral therapies are available, so survival depends on reversal of the underlying immunosuppression. Antiretroviral therapies greatly reduce the risk of HIV-related PML, but many modern treatments for cancers, organ transplantation and chronic inflammatory disease cause immunosuppression that can be difficult to reverse. These treatments — most notably natalizumab for multiple sclerosis — have led to a surge of iatrogenic PML. The spectrum of presentations of JCV- related disease has evolved over time and may challenge current diagnostic criteria. Immunotherapeutic interventions, such as use of checkpoint inhibitors and adoptive T cell transfer, have shown promise but caution is needed in the management of immune reconstitution inflammatory syndrome, an exuberant immune response that can contribute to morbidity and death. Many people who survive PML are left with neurological sequelae and some with persistent, low-level viral replication in the CNS. As the number of people who survive PML increases, this lack of viral clearance could create challenges in the subsequent management of some underlying diseases. Progressive multifocal leukoencephalopathy (PML) is for multiple sclerosis. Taken together, HIV, lymphopro- a rare, debilitating and often fatal disease of the CNS liferative disease and multiple sclerosis account for the caused by JC virus (JCV). -

Congenital Cytomegalovirus Infection Alters Olfaction Before Hearing Deterioration in Mice
10424 • The Journal of Neuroscience, December 5, 2018 • 38(49):10424–10437 Development/Plasticity/Repair Congenital Cytomegalovirus Infection Alters Olfaction Before Hearing Deterioration In Mice X Franc¸oise Lazarini,1,2 Lida Katsimpardi,1,2* Sarah Levivien,1,2,3*Se´bastien Wagner,1,2 XPierre Gressens,3,4,5 Natacha Teissier,3,4,6† and Pierre-Marie Lledo1,2† 1Institut Pasteur, Perception and Memory Unit, F-75015 Paris, France, 2Centre National de la Recherche Scientifique, Unite´ Mixte de Recherche 3571, F-75015 Paris, France, 3PROTECT, INSERM, Unite´ 1141, F-75019 Paris, France, 4Paris Diderot University, Sorbonne Paris Cite´, F-75018 Paris, France, 5Center for Developing Brain, King’s College, London, WC2R2LS United Kingdom, and 6Pediatric Otorhinolaryngology Department, Robert Debre´ Hospital, Assistance Publique–Hoˆpitaux de Paris, F-75019 Paris, France In developed countries, cytomegalovirus (CMV)-infected newborns are at high risk of developing sensorineural handicaps such as hearing loss, requiring extensive follow-up. However, early prognostic tools for auditory damage in children are not yet available. In the fetus, CMV infection leads to early olfactory bulb (OB) damage, suggesting that olfaction might represent a valuable prognosis for neurological outcome of this viral infection. Here, we demonstrate that in utero CMV inoculation causes fetal infection and growth retardation in mice of both sexes. It disrupts OB normal development, leading to disproportionate OB cell layers and rapid major olfactory deficits. Olfaction is impaired as early as day 6 after birth in both sexes, long before the emergence of auditory deficits. Olfactometry in males reveals a long-lasting alteration in olfactory perception and discrimination, particularly in binary mixtures of monomolecular odorants. -

Encephalitis Fact Sheet
Encephalitis Fact Sheet What is encephalitis? Encephalitis is an inflammation (irritation and swelling) of the brain tissue. It can occur as a primary illness or as a result of another illness elsewhere in the body. Frequently caused by an infection with a virus, encephalitis can be mild or severe, but severe cases are rare. Who gets encephalitis? Anyone can get encephalitis, but young children, older adults, and persons with weakened immune systems are more at risk. How is encephalitis spread? Primary encephalitis occurs when a virus directly infects the brain. Viruses that are carried by infected mosquitoes and ticks can cause primary encephalitis. Other viruses can cause an illness and then encephalitis can develop as a complication of the viral illness. When encephalitis develops after another infection, it is called post-infectious encephalitis. Post-infectious encephalitis most commonly follows a bout of chickenpox, mumps, or measles. What are the symptoms of encephalitis? Encephalitis frequently causes headache, fever, confused thinking, seizures, or problems with vision, speech, or movement. Infants and young children might have nausea, vomiting, body stiffness, constant crying, and poor feeding. How soon after exposure do symptoms appear? Encephalitis can occur within two to three weeks after a viral illness. Symptoms begin within a few days to one or two weeks after the bite from an infected mosquito. How is encephalitis diagnosed? Medical evaluation and special tests are needed to confirm the diagnosis. Anyone with a severe headache, fever, and altered mental status should seek medical care immediately. What is the treatment for encephalitis? Doctors might prescribe antiviral drugs, anti-inflammatory drugs, or other treatments for the symptoms as needed. -
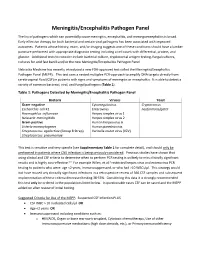
Meningitis/Encephalitis Pathogen Panel
Meningitis/Encephalitis Pathogen Panel The list of pathogens which can potentially cause meningitis, encephalitis, and meningoencephalitis is broad. Early effective therapy for both bacterial and certain viral pathogens has been associated with improved outcomes. Patients whose history, exam, and/or imaging suggests one of these conditions should have a lumber puncture performed with appropriate diagnostic testing including a cell count with differential, protein, and glucose. Additional tests to consider include bacterial culture, cryptococcal antigen testing, fungal cultures, cultures for acid fast bacilli and/or the new Meningitis/Encephalitis Pathogen Panel. Nebraska Medicine has recently introduced a new FDA-approved test called the Meningitis/Encephalitis Pathogen Panel (MEPP). This test uses a nested multiplex PCR-approach to amplify DNA targets directly from cerebrospinal fluid (CSF) in patients with signs and symptoms of meningitis or encephalitis. It is able to detect a variety of common bacterial, viral, and fungal pathogens (Table 1). Table 1: Pathogens Detected by Meningitis/Encephalitis Pathogen Panel Bacteria Viruses Yeast Gram-negative Cytomegalovirus Cryptococcus Escherichia coli K1 Enterovirus neoformans/gattii Haemophilus influenzae Herpes simplex virus 1 Neisseria meningitidis Herpes simplex virus 2 Gram-positive Human herpesvirus 6 Listeria monocytogenes Human parechovirus Streptococcus agalactiae (Group B Strep) Varicella zoster virus (VZV) Streptococcus pneumoniae This test is sensitive and very specific (see Supplementary Table 1 for complete detail), and should only be performed in patients where CNS infection is being seriously considered. Previous studies have shown that using clinical and CSF criteria to determine when to perform PCR testing is unlikely to miss clinically significant results and is highly cost-effective.1-3 For example Wilen, et al.3 restricted herpes virus and enterovirus PCR testing to patients who were: age <2 years, immunosuppressed, or who had >10 WBCs/µl. -

The Role of Herpes Simplex Virus Type 1 Infection in Demyelination of the Central Nervous System
International Journal of Molecular Sciences Review The Role of Herpes Simplex Virus Type 1 Infection in Demyelination of the Central Nervous System Raquel Bello-Morales 1,2,* , Sabina Andreu 1,2 and José Antonio López-Guerrero 1,2 1 Departamento de Biología Molecular, Universidad Autónoma de Madrid, Cantoblanco, 28049 Madrid, Spain; [email protected] (S.A.); [email protected] (J.A.L.-G.) 2 Centro de Biología Molecular Severo Ochoa, CSIC-UAM, Cantoblanco, 28049 Madrid, Spain * Correspondence: [email protected] Received: 30 June 2020; Accepted: 15 July 2020; Published: 16 July 2020 Abstract: Herpes simplex type 1 (HSV-1) is a neurotropic virus that infects the peripheral and central nervous systems. After primary infection in epithelial cells, HSV-1 spreads retrogradely to the peripheral nervous system (PNS), where it establishes a latent infection in the trigeminal ganglia (TG). The virus can reactivate from the latent state, traveling anterogradely along the axon and replicating in the local surrounding tissue. Occasionally, HSV-1 may spread trans-synaptically from the TG to the brainstem, from where it may disseminate to higher areas of the central nervous system (CNS). It is not completely understood how HSV-1 reaches the CNS, although the most accepted idea is retrograde transport through the trigeminal or olfactory tracts. Once in the CNS, HSV-1 may induce demyelination, either as a direct trigger or as a risk factor, modulating processes such as remyelination, regulation of endogenous retroviruses, or molecular mimicry. In this review, we describe the current knowledge about the involvement of HSV-1 in demyelination, describing the pathways used by this herpesvirus to spread throughout the CNS and discussing the data that suggest its implication in demyelinating processes. -

Intrathecal Antibody Production Against Epstein-Barr, Herpes Simplex, and Other Neurotropic Viruses in Autoimmune Encephalitis
ARTICLE OPEN ACCESS Intrathecal Antibody Production Against Epstein-Barr, Herpes Simplex, and Other Neurotropic Viruses in Autoimmune Encephalitis Philipp Schwenkenbecher, MD, Thomas Skripuletz, MD, Peter Lange, Marc Durr,¨ MD, Felix F. Konen, MD, Correspondence Nora Mohn,¨ MD, Marius Ringelstein, MD, Til Menge, MD, Manuel A. Friese, MD, Nico Melzer, MD, Dr. Schwenkenbecher schwenkenbecher.philipp@ ¨ Michael P. Malter, MD, Martin Hausler, MD, Franziska S. Thaler, MD, Martin Stangel, MD, Jan Lewerenz, MD, mh-hannover.de and Kurt-Wolfram Suhs,¨ MD, on behalf of the German Network for Research on Autoimmune Encephalitis Neurol Neuroimmunol Neuroinflamm 2021;8:e1062. doi:10.1212/NXI.0000000000001062 Abstract Background and Objectives Neurotropic viruses are suspected to play a role in the pathogenesis of autoimmune diseases of the CNS such as the association between the Epstein-Barr virus (EBV) and multiple sclerosis (MS). A group of autoimmune encephalitis (AE) is linked to antibodies against neuronal cell surface proteins. Because CNS infection with the herpes simplex virus can trigger anti–NMDA receptor (NMDAR) encephalitis, a similar mechanism for EBV and other neurotropic viruses could be postulated. To investigate for previous viral infections of the CNS, intrathecally produced virus-specific antibody synthesis was determined in patients with AE. Methods Antibody-specific indices (AIs) against EBV and measles, rubella, varicella zoster, herpes simplex virus, and cytomegalovirus were determined in 27 patients having AE (anti-NMDAR encephalitis, n = 21, and LGI1 encephalitis, n = 6) and in 2 control groups comprising of 30 patients with MS and 21 patients with noninflammatory CNS diseases (NIND), which were sex and age matched. Results An intrathecal synthesis of antibodies against EBV was found in 5/27 (19%) patients with AE and 2/30 (7%) of the patients with MS. -

Neurotropic Viral Infections in Bangladesh: Burden and Challenges
http://www.banglajol.info/index.php/BJID/index Editorial Bangladesh Journal of Infectious Diseases June 2016, Volume 3, Number 1 ISSN (Online) 2411-670X ISSN (Print) 2411-4820 Neurotropic Viral Infections in Bangladesh: Burden and Challenges Mohammad Enayet Hussain Assistant Professor, Department of Neurology, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh; Email: [email protected] Neurotropic virus infections continue to cause encephalopathies. In addition, influenza viruses major disease and economic burdens on society1. It have been linked to the development of Guillan poses a major challenge to human health care Barré syndrome, Kleine Levin syndrome and systems due to the associated morbidity and transfer myelitis5. Maternal influenza has been mortality worldwide. This creates a unique problem associated with schizophrenia and bipolar disorder in providing treatment to the patients involved. This (BD) in the offspring. Acute demyelinating is largely due to unique features of the central encephalomyelitis (ADME) typically occurs in nervous system (CNS), with a plethora of measles patients. Subacute sclerosing interconnected and interdependent cell types, panencephalitis (SSPE) occurs on average 4– complex structures and functions, reduced immune 10 years following acute MV infection. Mumps surveillance and limited regeneration capacity. virus was the leading cause of aseptic meningitis in Infection by neurotropic viruses as well as the local the pre-vaccine era. Pathologically, mumps induced immune responses can -

Cns Response in a Rabid Brain: a Pathological View
Review Article ISSN 2250-0480 VOL 5/ ISSUE 4/OCT 2015 CNS RESPONSE IN A RABID BRAIN: A PATHOLOGICAL VIEW 1PREETY SHARMA, 1 SANJEEV KUMAR,2 V. K. SHARMA* & AJAY KUMAR TEHLAN 1Central Research Institute, Kasauli, District Solan, Himachal Pradesh -173204, India. 2Department of Pharmacology, Govt. College of Pharmacy, Rohru, Distt. Shimla, Himachal Pradesh-171207, India ABSTRACT Animal attacks constitute a huge medical and social problem ending in millions of injuries and thousands of deaths worldwide. Rabies is a dreadful infectious disease that has not been brought under control in many parts of the world even today. Rabies spread by domestic and wild animals particularly by a dog bite, and with the exception of Antarctica, rabies is present on all continents, taking a heavy toll on human lives. Many countries have the status of high-risk areas, but most of the countries around the globe gained the status of rabies free territories. This shows that rabies can be successfully ruled out from the high-risk areas by taking preventing measures and the situation may be better if we continue to untangle the mysterious pathophysiology of this disease. Understanding rabies invasion and its pathophysiology will further enhance the chances of improvement rabies related complications and mortality. Rabies is a prototypic infection of the nervous system in which the virus selectively infects neurons, using retrograde axonal transport to traffic in the nervous system. Neuroinvasiveness, neurotropism and neurovirulence are the major defining characteristics of this virus. The speed of virus uptake, the ability of the virus to spread efficiently from cell-to-cell and the rate of virus replication are the major factors that determine the pathogenicity of rabies virus.